The efficacy and safety of oral antibiotic treatment in patients with chronic low back pain and Modic changes: A systematic review and meta-analysis
Abstract
Background
This systematic review and meta-analysis aimed to summarize evidence regarding the effectiveness and safety of oral antibiotic intervention for chronic low back pain (CLBP) patients with/without type-1 Modic changes (MC1).
Methods
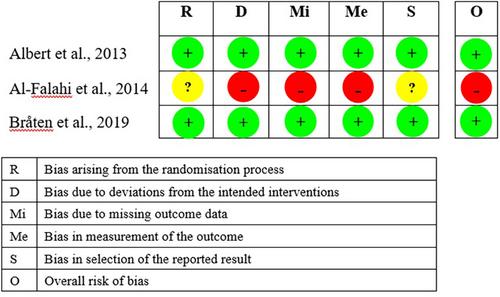
AMED, CINAHL, Cochrane Library, Embase, and Medline were searched from inception to March 3, 2023. Randomized controlled trials (RCTs) or non-RCTs that investigated the effectiveness or safety of oral antibiotics in treating CLBP patients were eligible for inclusion. Two independent reviewers screened abstracts, full-text articles, and extracted data. The methodological quality of each included article were evaluated by RoB2 and NIH quality assessment tools. The quality of evidence was appraised by GRADE. Meta-analyses were performed, where applicable. A subgroup analysis was conducted to evaluate the RCTs and case series separately, and to evaluate the effect of removing a low-quality RCT.
Results
Three RCTs and four case series were included. All Amoxicillin-clavulanate/Amoxicillin treatments lasted for approximately 3 months. Moderate- and low-quality evidence suggested that antibiotic was significantly better than placebo in improving disability and quality of life in CLBP patients with MC1 at 12-month follow-up, respectively. Low-quality evidence from meta-analyses of RCTs showed that oral antibiotic was significantly better than placebo in improving pain and disability in CLBP patients with MC1 immediately post-treatment. Very low-quality evidence from the case series suggested that oral Amoxicillin-clavulanate significantly improved LBP/leg pain, and LBP-related disability. Conversely, low-quality evidence found that oral Amoxicillin alone was not significantly better than placebo in improving global perceived health in patients with CLBP at the 12-month follow-up. Additionally, oral antibiotic users had significantly more adverse effects than placebo users.
Conclusions
Although oral antibiotics were statistically superior to placebo in reducing LBP-related disability in patients with CLBP and concomitant MC1, its clinical significance remains uncertain. Future large-scale high-quality RCTs are warranted to validate the effectiveness of antibiotics in individuals with CLBP.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


