Rh(iii)-catalyzed redox-neutral C–H [4 + 1] annulation of sulfoximines with α,α-difluoromethylene alkynes: diastereoselective synthesis of E-monofluoroalkenyl benzoisothiazole 1-oxides†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Novel cyclic fluorinated sulfoximines featuring an E-monofluoroalkenyl benzoisothiazole 1-oxide moiety as single diastereomers can be facilely accessed by Rh(III)-catalyzed redox-neutral [4 + 1] annulation of sulfoximines with α,α-difluoromethylene alkynes. The reaction proceeds with sequential selective cleavage of both C–H and C–F bonds, thus exhibiting high step and atom economy. Through a combined experimental and computational mechanistic study, the origins of annulative chemoselectivity, unconventional E-selectivity, and excellent diastereoselectivity have been revealed accordingly.
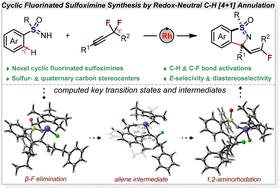
Rh(III)催化亚砜亚胺与α,α-二氟乙烯的氧化还原-中性C-H[4+1]环化:e -单氟烯基苯并异噻唑1-氧化物的非对映选择性合成
以e -单氟烯基苯并异噻唑1-氧化物为单对映体的新型环氟亚胺可以通过Rh(III)催化氧化还原-中性[4+1]环亚胺与α,α-二氟亚甲基炔的反应得到。反应继续…
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


