Regioselective Synthesis of 2,4- and 2,5-disubstituted 1,3-thiazoles from 2-oxo-2-(amino)ethanedithioates via Base Catalysed Cyclization
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We herein report efficient methods for synthesis of 2,4- and 2,5-disubstituted-1,3-thiazoles 3(a-i) and 5(a-k) by the cyclization of 2-oxo-2-(amino)ethanedithioates with TosMIC and α-haloketones in high yields. The structures 3a and 5a were confirmed based on X-ray crystallographic studies. In addition, investigation of ground state geometry, electronic and molecular structural properties, FMOs, global reactivity descriptors, MEP and NCI analyses predicted to access the information related to the stability, reactivity, and strength of the interactions present in the molecules by quantum chemical calculations. Further, the potency of derivatives tested against the SARS-Cov2 receptor (PDB ID: 7mc6) via molecular docking approach with binding scores of –6.0 to –8.4 kcal/mol.
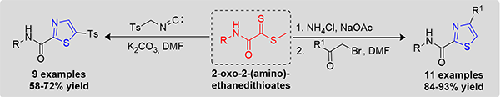
碱基催化环化2-氧-2-(氨基)乙二硫酸酯合成2,4-和2,5-二取代1,3-噻唑的区域选择性
本文报道了用2-氧-2-(氨基)乙二硫代酸酯与TosMIC和α-卤酮环化合成2,4-和2,5-二取代-1,3-噻唑3(a-i)和5(a-k)的高效方法。x射线晶体学研究证实了结构3a和5a。此外,对基态几何、电子和分子结构性质、FMOs、全局反应性描述符、MEP和NCI分析的研究预测,通过量子化学计算可以获得与分子中存在的相互作用的稳定性、反应性和强度相关的信息。此外,通过分子对接方法测试了衍生物对SARS-Cov2受体(PDB ID: 7mc6)的效价,结合评分为-6.0至-8.4 kcal/mol。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


