Kinetic Analysis of Hydrogen Reduction of Nickel Compounds
IF 3.1
2区 材料科学
Q3 MATERIALS SCIENCE, MULTIDISCIPLINARY
Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science
Pub Date : 2023-11-14
DOI:10.1007/s11663-023-02955-6
引用次数: 0
Abstract
Abstract A review on the hydrogen reduction kinetics of NiO, NiCO 3, and Ni(OH) 2 hydrogen was conducted, and the most significant experimental values and results were summarized from the past two decades. Isothermal hydrogen reduction experiments of NiO, NiCO 3, and, Ni(OH) 2 experiments were carried out at 300 °C, 400 °C, and 500 °C, and the obtained data were fitted to multiple different solid-state kinetic models in order to compare the suitability of the models. Non-isothermal reduction in H 2 (0 °C $$\to $$
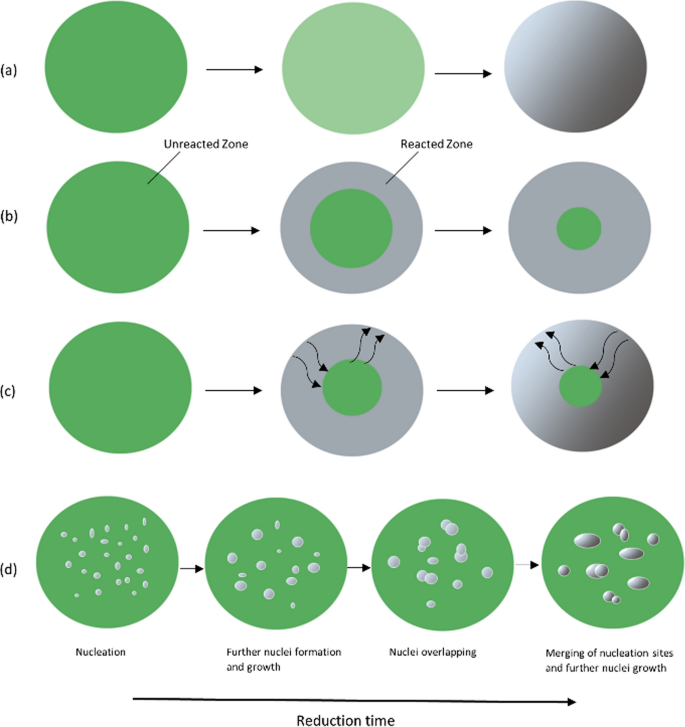
氢还原镍化合物的动力学分析
摘要对NiO、NiCO 3和Ni(OH) 2氢的氢还原动力学进行了综述,总结了近二十年来最重要的实验值和结果。分别在300°C、400°C和500°C条件下对NiO、NiCO 3和Ni(OH) 2进行等温氢还原实验,并将所得数据拟合到多个不同的固态动力学模型中,比较模型的适用性。在h2(0℃$$\to $$→900℃)中非等温还原,nico3在230℃~ 300℃分解,Ni(OH) 2在220℃~ 300℃分解。nico3、Ni(OH) 2和NiO的ea值分别在30 ~ 35,36 ~ 37,21 ~ 26kj /mol之间变化。大多数使用的模型都很好地拟合,使得明确选择最合适的模型变得困难,并确定了反应背后的机理。结果表明,温度的升高加速了还原过程,反应速率的控制机理有待进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

CiteScore
4.90
自引率
20.00%
发文量
293
审稿时长
4.7 months
期刊介绍:
Focused on process metallurgy and materials processing science, Metallurgical and Materials Transactions B contains only original, critically reviewed research on primary manufacturing processes, from extractive metallurgy to the making of a shape.
A joint publication of ASM International and TMS (The Minerals, Metals and Materials Society), Metallurgical and Materials Transactions B publishes contributions bimonthly on the theoretical and engineering aspects of the processing of metals and other materials, including studies of electro- and physical chemistry, mass transport, modeling and related computer applications.
Articles cover extractive and process metallurgy, pyrometallurgy, hydrometallurgy, electrometallurgy, transport phenomena, process control, physical chemistry, solidification, mechanical working, solid state reactions, composite materials, materials processing and the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


