A Radical-Initiated Fragmentary Rearrangement Cascade of Ene-Ynamides to [1,2]-Annulated Indoles via Site-Selective Cyclization
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 5
Abstract
Straightforward access to [1,2]-annulated indoles, key substructures in natural products, is highly desirable yet challenging. Herein, a radical triggered fragmentary cyclization cascade reaction of ene-ynamides is presented, providing a rapid access into [1,2]-annulated indoles by an intermolecular radical addition, intramolecular cyclization, desulfonylative aryl migration, and site-selective C(sp2)-N cyclization sequence. DFT calculations support oxidation of N-centered radical species to cations prior to the C–N bond formation, followed by an unusual aza-Nazarov cyclization.
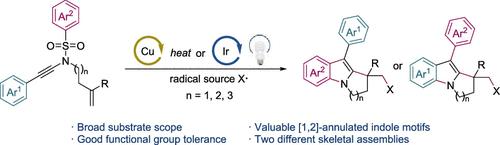
通过位点选择性环化,自由基引发的烯酰胺到[1,2]环状吲哚的片段重排级联
直接获取天然产物中的关键亚结构[1,2]环吲哚是非常可取的,但也具有挑战性。本文提出了一种由自由基触发的烯酰酰胺片段环化级联反应,通过分子间自由基加成、分子内环化、芳基迁移和位点选择性C(sp2)-N环化序列,快速进入[1,2]环化吲哚。DFT计算支持在C-N键形成之前,n中心自由基氧化成阳离子,然后是不寻常的aza-Nazarov环化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


