Palladium and Nickel Catalyzed Suzuki Cross-Coupling with Alkyl Fluorides
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 6
Abstract
Suzuki cross-coupling of benzylic and unactivated aliphatic fluorides with aryl- and alkenylboronic acids has been achieved via mechanistically distinct Pd and Ni catalyzed pathways that outperform competing protodeboronation, β-hydride elimination, and homocoupling processes. The utility is demonstrated with more than 20 examples including heterocyclic structures, 1,1-disubstituted and trans-1,2-disubstituted alkenes, and by the incorporation of acetonitrile into functionalized (hetero)arenes.
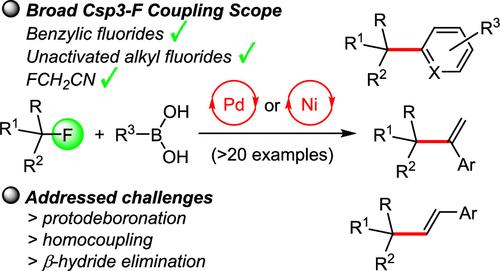
钯镍催化氟烷基化合物铃木交联反应
苯基和未活化的脂肪族氟化物与芳基和烯基硼酸的铃木交叉偶联是通过机制不同的Pd和Ni催化途径实现的,其效果优于竞争性的原deboronation、β-氢化物消除和均偶联过程。该实用程序通过20多个示例进行了演示,包括杂环结构,1,1-二取代和反-1,2-二取代烯烃,以及将乙腈掺入功能化(杂)芳烃中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


