Photoredox-Catalyzed Difunctionalization of Unactivated Olefins for Synthesizing Lactam-Substituted gem-Difluoroalkenes
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 17
Abstract
Herein, the synthesis of lactam-substituted gem-difluoroalkenes has been developed through a photoredox-catalyzed radical cascade reaction. This developed photoredox-catalyzed, Br?nsted base-assisted intramolecular 5-exo-trig cyclization/intermolecular radical addition/β-fluoride elimination reaction offers a simple method for producing lactam, carbamate, or urea-substituted gem-difluoroalkenes with good functional group tolerance and high yields.
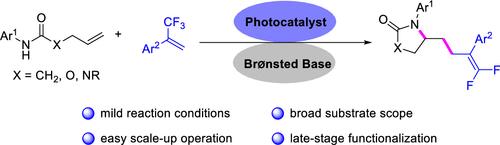
光氧化还原催化非活化烯烃双官能化合成内酰胺取代宝石二氟烯烃
本文通过光氧化还原催化自由基级联反应合成了内酰胺取代的宝石二氟烯烃。这产生了光氧化还原催化的Br?Nsted碱基辅助分子内5-外三角环化/分子间自由基加成/β-氟消除反应为生产具有良好官能团耐受性和高产率的内酰胺、氨基甲酸酯或脲取代宝石二氟烯烃提供了一种简单的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


