Analisi costo-efficacia di caplacizumab nel nuovo standard of care della porpora trombotica trombocitopenica immune in Italia.
IF 0.4
Q4 HEALTH CARE SCIENCES & SERVICES
引用次数: 3
Abstract
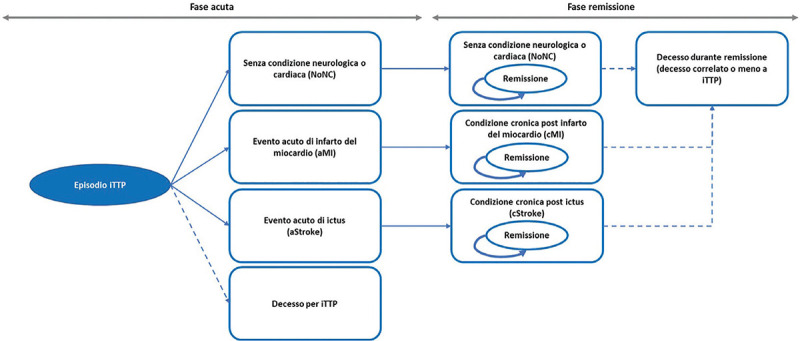
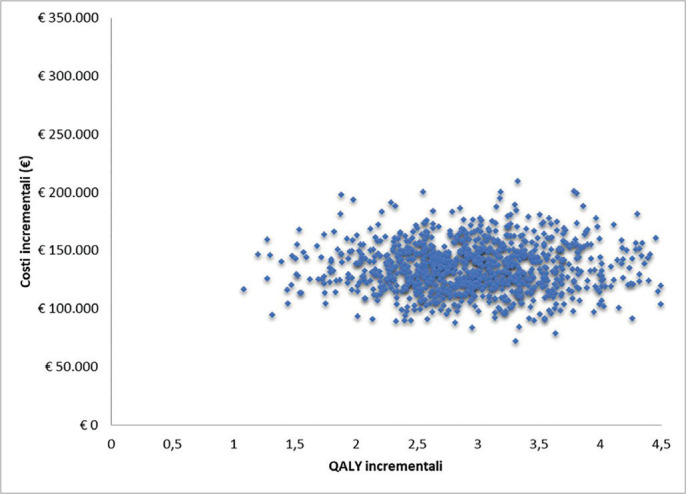
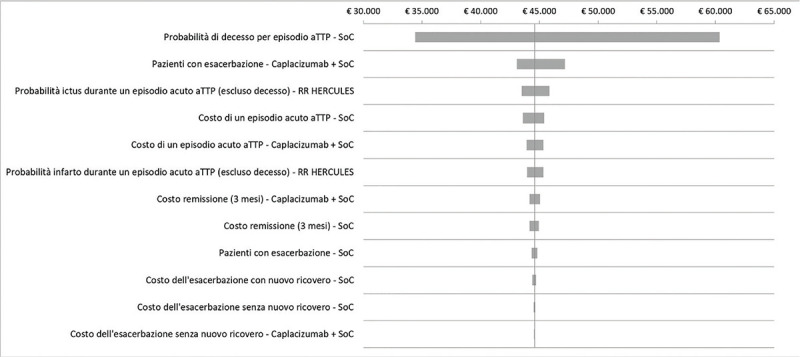
Cost-effectiveness analysis of caplacizumab in the new standard of care for immune Thrombotic Thrombocytopenic Purpura in Italy Objectives: To evaluate the cost-effectiveness analysis (CEA) of caplacizumab in combination with plasmapheresis (PEX) and immunosuppression compared to PEX and immunosuppression in the treatment of acute episodes of iTTP. Methods: A Markov model was used to conduct the CEA from the perspective of the hospital, over a lifetime horizon. Clinical data derived from HERCULES trial and a systematic literature review. Economic input included direct costs only. Utility and disutility values were obtained from literature. Data on healthcare resources and costs were retrieved from HERCULES trial, literature, TTP guidelines and Italian tariffs. A sensitivity analysis was conducted. The cost-effectiveness probability was tested for several options of discount levels considering a suggested willingness to pay (WTP) threshold of € 60,000 in Italy. Results: The use of caplacizumab in combination with PEX and immunosuppression is associated with a positive difference in survival of 3.27 life years (24.53 vs 21.26) and in quality of life of 3.06 QALY (22.01 vs 18.96) when compared to PEX and immunosuppression. Caplacizumab leads to an ICER per life years of € 41,653 and an ICER per QALY of € 44,572. For the suggested WTP threshold, the probability of caplacizumab being cost-effective is 82.4% (no discount), 92.8% (15% discount), 95.3% (20% discount), 96.9% (25% discount) and 98.2% (30% discount). Conclusions: Caplacizumab in addition to PEX and immunosuppression is cost-effective, allowing the hospital to achieve greater efficiency in managing the burden of a life-threatening disease such as iTTP.
意大利免疫血小板减少紫癜新护理标准中的caplacizumab的成本效益分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Global & Regional Health Technology Assessment
HEALTH CARE SCIENCES & SERVICES-
CiteScore
0.80
自引率
20.00%
发文量
27
审稿时长
8 weeks
期刊介绍:
Global & Regional Health Technology Assessment (GRHTA) is a peer-reviewed, open access journal which aims to promote health technology assessment and economic evaluation, enabling choices among alternative therapeutical paths or procedures with different clinical and economic outcomes. GRHTA is a unique journal having three different editorial boards who focus on their respective geographical expertise.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


