Costo per responder di upadacitinib e abatacept nel trattamento dell’artrite reumatoide da moderata a grave in Italia.
IF 0.4
Q4 HEALTH CARE SCIENCES & SERVICES
引用次数: 1
Abstract
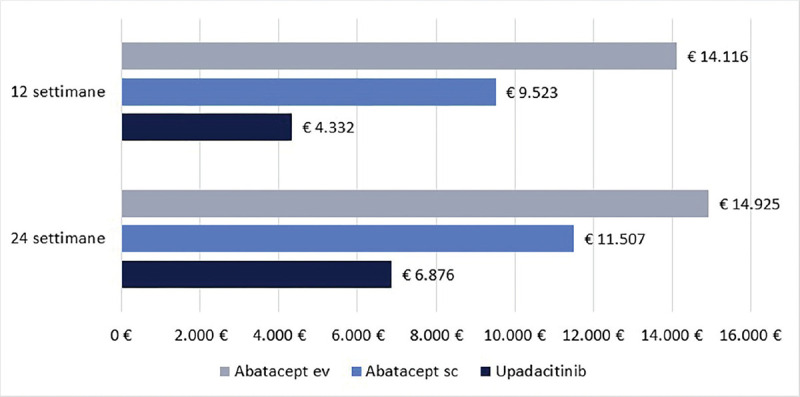
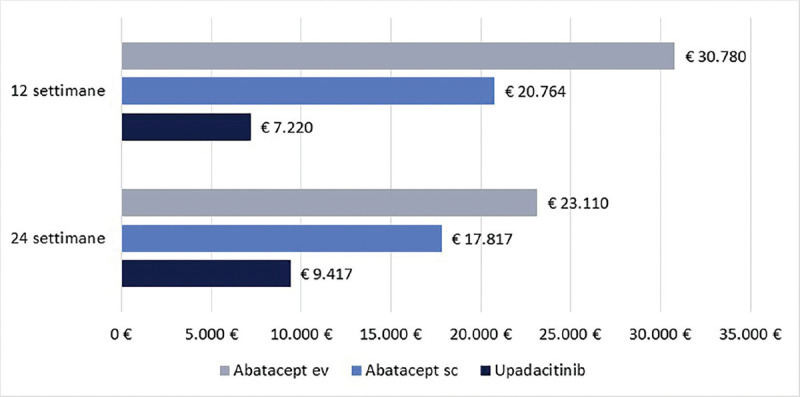
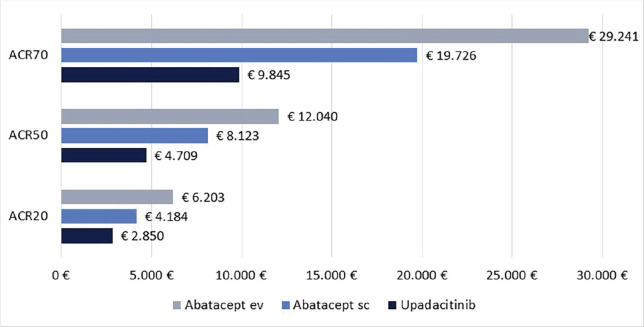
Cost per responder for upadacitinib vs abatacept in patients with moderate-to-severe Rheumatoid Arthritis in Italy Purpose: The objective of this economic evaluation was to compare the cost per responder between upadacitinib and abatacept (intravenous [iv] or subcutaneous [sc]) in patients with moderate-to-severe Rheumatoid Arthritis (RA) in Italy. Methods: The clinical efficacy was assessed based on SELECT-CHOICE study results. The clinical efficacy of upadacitinib and abatacept (iv or sc) was measured by Clinical Remission (CR), Low Disease Activity (LDA) and American College of Rheumatology response (ACR20, 50 and 70). The treatment cost was based on the number of administrations dispensed at 12 or 24 weeks. The cost per responder was adopted as a cost-effectiveness indicator. Results: Independent of the clinical efficacy measure used and the duration of treatment considered, the cost per responder was consistently lower for upadacitinib compared to abatacept (iv or sc) across all clinical measures. For example, considering the CR at 24 weeks, the cost per responder for upadacitinib was € 9,417 compared to € 17,817 for abatacept sc or to € 23,110 for abatacept iv. The differences in the cost per responder between upadacitinib and abatacept (iv or sc) increased when higher ACR response levels were considered. Conclusions: These results suggested that upadacitinib is a cost-effectiveness option compared to abatacept (iv or sc) from the perspective of the Italian National Health Service in patients with moderate-to-severe Rheumatoid Arthritis in Italy.
在意大利,upadacitinib和abatacept治疗风湿性关节炎中度到严重关节炎的费用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Global & Regional Health Technology Assessment
HEALTH CARE SCIENCES & SERVICES-
CiteScore
0.80
自引率
20.00%
发文量
27
审稿时长
8 weeks
期刊介绍:
Global & Regional Health Technology Assessment (GRHTA) is a peer-reviewed, open access journal which aims to promote health technology assessment and economic evaluation, enabling choices among alternative therapeutical paths or procedures with different clinical and economic outcomes. GRHTA is a unique journal having three different editorial boards who focus on their respective geographical expertise.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


