Cryo-electron microscopy structures of human thyroid peroxidase (TPO) in complex with TPO antibodies.
IF 3.8
4区 医学
Q2 ENDOCRINOLOGY & METABOLISM
引用次数: 1
Abstract
Determination of the structure of the extracellular domain of human thyroid peroxidase (hTPO) by cryo-electron microscopy (cryo-EM) is described. TPO, purified to homogeneity was complexed with the hTPO monoclonal autoantibody 2G4 Fab and also with a mouse monoclonal TPO antibody 4F5 Fab (which competes with autoantibody binding to TPO). Both complexes were analysed by cryo-EM. The two structures (global resolution 3.92 and 3.4 Å for the 2G4 complex and 4F5 complex, respectively) show TPO as a monomer with four domains; the N-terminal domain, the peroxidase domain (POD), the complement control protein (CCP)-like domain and the epidermal growth factor-like domain which are all visible in the structures. The relative positions of the domains are fixed with a disulphide bond between cysteine residues Cys146 in the POD and Cys756 in the CCP domain preventing significant flexibility of the molecule. The entrance to the enzyme active site, the haem group and the calcium binding site are clearly visible on the opposite side of the TPO molecule from the 2G4 and 4F5 binding sites. Extensive interactions are seen between TPO and the two antibodies which both bind to distinct epitopes on the POD domain, including some residues in the immunodominant region B mainly via different residues. However, the epitopes of the two antibodies contain three shared TPO residues. This is the first high-resolution structure of TPO to be reported and it should help guide the development of new inhibitors of TPO enzyme activity for therapeutic applications.

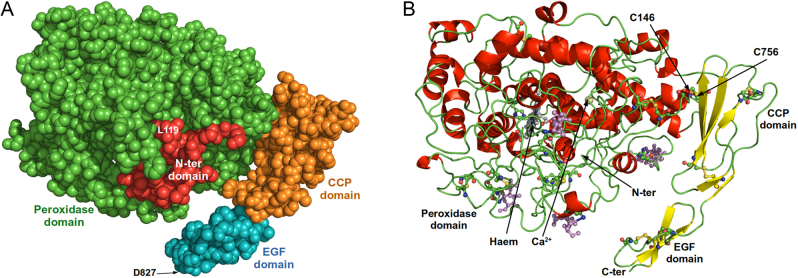
人甲状腺过氧化物酶(TPO)与TPO抗体复合物的低温电镜结构。
描述了用冷冻电镜(cryo-EM)测定人甲状腺过氧化物酶(hTPO)细胞外结构域的结构。纯化至均匀的TPO与hTPO单克隆自身抗体2G4 Fab和小鼠单克隆TPO抗体4F5 Fab(与TPO自身抗体竞争)络合。两种配合物均通过低温电镜分析。这两个结构(2G4配合物和4F5配合物的全局分辨率分别为3.92和3.4 Å)表明TPO是一个具有四个结构域的单体;n端结构域,过氧化物酶结构域(POD),补体控制蛋白(CCP)样结构域和表皮生长因子样结构域都在结构中可见。这些结构域的相对位置是固定的,在POD中的半胱氨酸残基Cys146和CCP结构域的Cys756之间有一个二硫键,阻止了分子的显著灵活性。在TPO分子与2G4和4F5结合位点相对的另一侧,酶活性位点、血红素基团和钙结合位点的入口清晰可见。TPO与两种抗体之间存在广泛的相互作用,这两种抗体都结合在POD结构域的不同表位上,包括免疫优势区B的一些残基,主要是通过不同的残基。然而,这两种抗体的表位含有三个共享的TPO残基。这是报道的第一个高分辨率的TPO结构,它应该有助于指导开发新的TPO酶活性抑制剂用于治疗应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of molecular endocrinology
医学-内分泌学与代谢
CiteScore
6.90
自引率
0.00%
发文量
96
审稿时长
1 months
期刊介绍:
The Journal of Molecular Endocrinology is an official journal of the Society for Endocrinology and is endorsed by the European Society of Endocrinology and the Endocrine Society of Australia.
Journal of Molecular Endocrinology is a leading global journal that publishes original research articles and reviews. The journal focuses on molecular and cellular mechanisms in endocrinology, including: gene regulation, cell biology, signalling, mutations, transgenics, hormone-dependant cancers, nuclear receptors, and omics. Basic and pathophysiological studies at the molecule and cell level are considered, as well as human sample studies where this is the experimental model of choice. Technique studies including CRISPR or gene editing are also encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


