Clinical utility of pharmacogenetics in a psychiatric and primary care population
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 3
Abstract
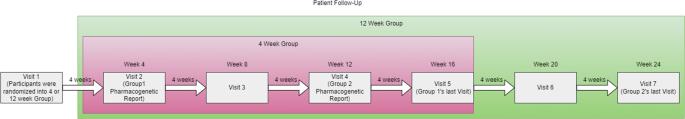
This study evaluated the timing, use, and clinical outcomes of the GeneFolio® Pharmacogenomic Panel in a healthcare setting with patients managed by primary care providers or by psychiatrists. Participants were randomized to receive a pharmacogenetics report at four weeks or 12 weeks. After DNA collection and genetic analysis, pharmacists produced a recommendation report which was given to providers at the randomization week. The four-week group decreased depression severity (PHQ-9 and BDI) faster than the 12-week group (p = 0.0196), and psychiatrists’ patients decreased their depression severity faster than primary care patients (PHQ-9 p = 0.0005, BDI p = 0.0218). Mean mental quality of life increased over time (p < 0.0001), but it increased slower for patients taking drugs in the Significant drug-drug-gene interaction category (p = 0.0012). Mental quality of life, depression severity, and clinical outcomes were improved by GeneFolio® pharmacogenomic testing regardless of provider type, with earlier testing improving outcomes sooner.
药物遗传学在精神科和初级保健人群中的临床实用性
这项研究评估了 GeneFolio® 药物基因组面板在医疗机构中的使用时间、使用情况和临床效果,研究对象是由初级保健提供者或精神科医生管理的患者。参与者被随机分配在四周或十二周时接受药物基因组学报告。经过 DNA 采集和基因分析后,药剂师会生成一份建议报告,在随机化的那一周交给医疗服务提供者。四周组抑郁严重程度(PHQ-9 和 BDI)的下降速度快于 12 周组(P = 0.0196),精神科患者抑郁严重程度的下降速度快于初级保健患者(PHQ-9 P = 0.0005,BDI P = 0.0218)。随着时间的推移,平均精神生活质量有所提高(p <0.0001),但服用药物-药物-基因相互作用显著类别药物的患者的精神生活质量提高较慢(p = 0.0012)。无论医疗机构的类型如何,GeneFolio®药物基因组学检测都能改善患者的精神生活质量、抑郁严重程度和临床疗效,而更早的检测能更快地改善疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


