Vasodilators activate the anion channel TMEM16A in endothelial cells to reduce blood pressure
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Systemic blood pressure is acutely controlled by total peripheral resistance as determined by the diameter of small arteries and arterioles, the contractility of which is regulated by endothelial cells lining the lumen of blood vessels. We investigated the physiological functions of the chloride (Cl−) channel TMEM16A in endothelial cells. TMEM16A channels generated calcium (Ca2+)–activated Cl− currents in endothelial cells from control (TMEM16Afl/fl) mice that were absent in those from mice with tamoxifen-inducible, endothelial cell–specific knockout of TMEM16A (TMEM16A ecKO). TMEM16A currents in endothelial cells were activated by the muscarinic receptor agonist acetylcholine and an agonist of the Ca2+ channel TRPV4, which localized in nanoscale proximity with TMEM16A as assessed by single-molecule localization imaging of endothelial cells. Acetylcholine stimulated TMEM16A currents by activating Ca2+ influx through surface TRPV4 channels without altering the nanoscale properties of TMEM16A and TRPV4 surface clusters or their colocalization. In pressurized arteries, activation of TMEM16A channels in endothelial cells induced by acetylcholine; TRPV4 channel stimulation; or intraluminal ATP, another vasodilator, produced hyperpolarization and dilation. Furthermore, deficiency of TMEM16A channels in endothelial cells resulted in increased systemic blood pressure in conscious mice. These data indicate that vasodilators stimulate TRPV4 channels, leading to Ca2+-dependent activation of nearby TMEM16A channels in endothelial cells to produce arterial hyperpolarization, vasodilation, and reduced blood pressure. Thus, TMEM16A is an anion channel in endothelial cells that regulates arterial contractility and blood pressure.
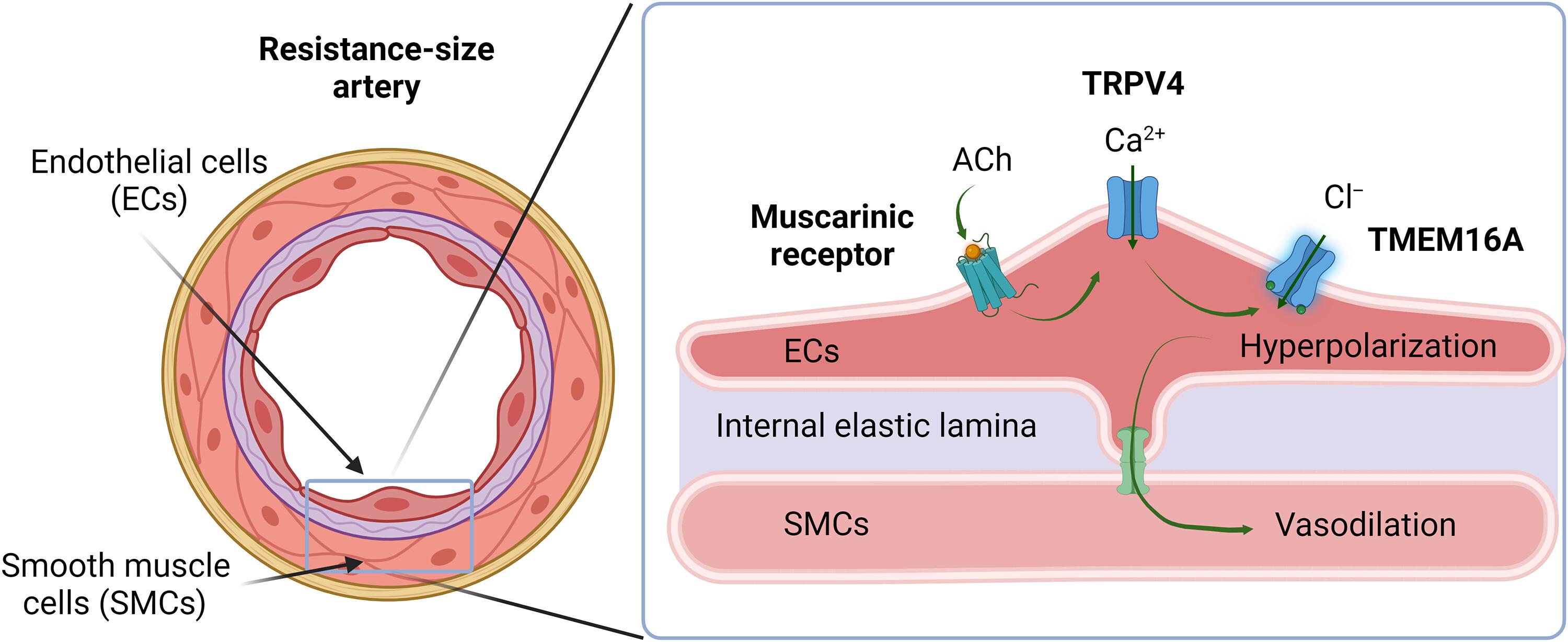
血管扩张剂激活内皮细胞中的阴离子通道TMEM16A以降低血压。
全身血压受到由小动脉和小动脉直径决定的总外周阻力的急性控制,小动脉和小动脉的收缩性由血管腔内的内皮细胞调节。我们研究了内皮细胞中氯离子通道TMEM16A的生理功能。TMEM16A通道在对照(TMEM16Afl/fl)小鼠的内皮细胞中产生钙(Ca2+)激活的Cl-电流,而在他莫昔芬诱导的内皮细胞特异性敲除TMEM16A (TMEM16A ecKO)小鼠的内皮细胞中则没有。内皮细胞中的TMEM16A电流被毒瘤碱受体激动剂乙酰胆碱和Ca2+通道激动剂TRPV4激活,通过内皮细胞的单分子定位成像评估,它们与TMEM16A在纳米尺度上接近。乙酰胆碱通过激活Ca2+内流通过表面TRPV4通道刺激TMEM16A电流,而不改变TMEM16A和TRPV4表面簇的纳米级性质或它们的共定位。在受压动脉中,乙酰胆碱诱导内皮细胞TMEM16A通道的激活;TRPV4通道刺激;或另一种血管扩张剂,腔内ATP,产生超极化和扩张。此外,内皮细胞中TMEM16A通道的缺乏导致清醒小鼠的全身血压升高。这些数据表明,血管扩张剂刺激TRPV4通道,导致内皮细胞中附近的TMEM16A通道的Ca2+依赖性激活,从而产生动脉超极化、血管舒张和降低血压。因此,TMEM16A是内皮细胞中的阴离子通道,调节动脉收缩性和血压。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


