Ferroptosis is critical for phthalates driving the blood-testis barrier dysfunction via targeting transferrin receptor
Abstract
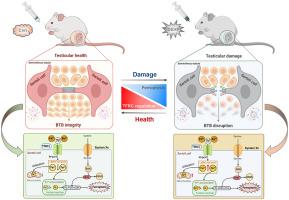
The global rate of human male infertility is rising at an alarming rate owing to environmental and lifestyle changes. Phthalates are the most hazardous chemical additives in plastics and have an apparently negative impact on the function of male reproductive system. Ferroptosis is a recently described form of iron-dependent cell death and has been linked to several diseases. Transferrin receptor (TfRC), a specific ferroptosis marker, is a universal iron importer for all cells using extracellular transferrin. We aim to investigate the potential involvement of ferroptosis during male reproductive toxicity, and provide means for drawing conclusions on the effect of ferroptosis in phthalates-induced male reproductive disease. In this study, we found that di (2-ethylhexyl) phthalate (DEHP) triggered blood-testis barrier (BTB) dysfunction in the mouse testicular tissues. DEHP also induced mitochondrial morphological changes and lipid peroxidation, which are manifestations of ferroptosis. As the primary metabolite of DEHP, mono-2-ethylhexyl phthalate (MEHP) induced ferroptosis by inhibiting glutathione defense network and increasing lipid peroxidation. TfRC knockdown blocked MEHP-induced ferroptosis by decreasing mitochondrial and intracellular levels of Fe2+. Our findings indicate that TfRC can regulate Sertoli cell ferroptosis and therefore is a novel therapeutic molecule for reproductive disorders in male patients with infertility.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


