Co-mutation pattern, clonal hierarchy, and clone size concur to determine disease phenotype of SRSF2P95-mutated neoplasms
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 10
Abstract
Somatic mutations in splicing factor genes frequently occur in myeloid neoplasms. While SF3B1 mutations are associated with myelodysplastic syndromes (MDS) with ring sideroblasts, SRSF2P95 mutations are found in different disease categories, including MDS, myeloproliferative neoplasms (MPN), myelodysplastic/myeloproliferative neoplasms (MDS/MPN), and acute myeloid leukemia (AML). To identify molecular determinants of this phenotypic heterogeneity, we explored molecular and clinical features of a prospective cohort of 279 SRSF2P95-mutated cases selected from a population of 2663 patients with myeloid neoplasms. Median number of somatic mutations per subject was 3. Multivariate regression analysis showed associations between co-mutated genes and clinical phenotype, including JAK2 or MPL with myelofibrosis (OR = 26.9); TET2 with monocytosis (OR = 5.2); RAS-pathway genes with leukocytosis (OR = 5.1); and STAG2, RUNX1, or IDH1/2 with blast phenotype (MDS or AML) (OR = 3.4, 1.9, and 2.1, respectively). Within patients with SRSF2–JAK2 co-mutation, JAK2 dominance was invariably associated with clinical feature of MPN, whereas SRSF2 mutation was dominant in MDS/MPN. Within patients with SRSF2–TET2 co-mutation, clinical expressivity of monocytosis was positively associated with co-mutated clone size. This study provides evidence that co-mutation pattern, clone size, and hierarchy concur to determine clinical phenotype, tracing relevant genotype–phenotype associations across disease entities and giving insight on unaccountable clinical heterogeneity within current WHO classification categories.
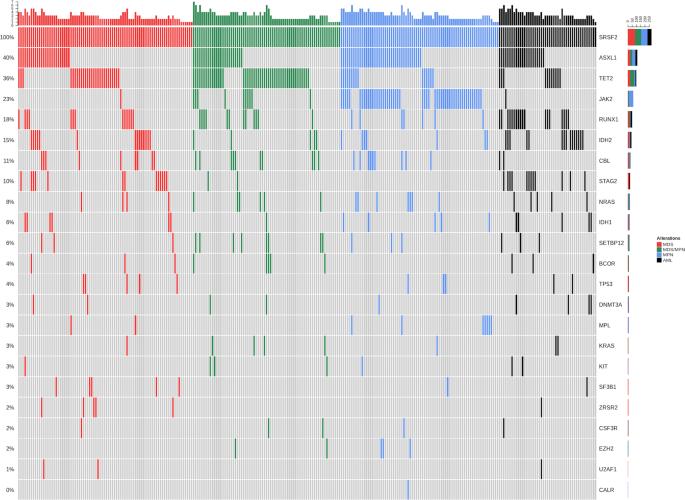
共突变模式、克隆层次和克隆大小共同决定了 SRSF2P95 突变肿瘤的疾病表型
剪接因子基因的体细胞突变经常发生在骨髓肿瘤中。SF3B1 基因突变与伴有环形红细胞的骨髓增生异常综合征(MDS)有关,而 SRSF2P95 基因突变则出现在不同的疾病类别中,包括骨髓增生异常综合征(MDS)、骨髓增生性肿瘤(MPN)、骨髓增生异常/骨髓增生性肿瘤(MDS/MPN)和急性髓系白血病(AML)。为了确定这种表型异质性的分子决定因素,我们对从2663名骨髓性肿瘤患者中筛选出的279例SRSF2P95突变病例的分子和临床特征进行了前瞻性研究。多变量回归分析显示,共同突变基因与临床表型之间存在关联,包括JAK2或MPL与骨髓纤维化(OR = 26.9);TET2与单核细胞增多症相关(OR = 5.2);RAS通路基因与白细胞增多症相关(OR = 5.1);STAG2、RUNX1或IDH1/2与爆破表型(MDS或AML)相关(OR分别为3.4、1.9和2.1)。在SRSF2-JAK2共突变患者中,JAK2显性与骨髓增生性疾病的临床特征相关,而SRSF2突变在MDS/骨髓增生性疾病中显性。在SRSF2-TET2共突变患者中,单核细胞增多症的临床表现与共突变克隆大小呈正相关。这项研究提供了共突变模式、克隆大小和层次结构共同决定临床表型的证据,追踪了跨疾病实体的相关基因型-表型关联,并对目前WHO分类类别中无法解释的临床异质性提出了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


