Effects of CYP2D6*10 allele on the pharmacokinetics of tolperisone
Abstract
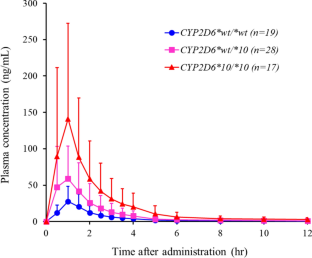
Tolperisone, a muscle relaxant used for post-stroke spasticity, has been reported to have a very wide interindividual pharmacokinetic variability. It is metabolized mainly by CYP2D6 and, to a lesser extent, by CYP2C19 and CYP1A2. CYP2D6 is a highly polymorphic enzyme, and CYP2D6*wt/*wt, CYP2D6*wt/*10 and CYP2D6*10/*10 genotypes constitute more than 90% of the CYP2D6 genotypes in the Korean population. Thus, effects of the CYP2D6*10 on tolperisone pharmacokinetics were investigated in this study to elucidate the reasons for the wide interindividual variability. Oral tolperisone 150 mg was given to sixty-four healthy Koreans, and plasma concentrations of tolperisone were measured by liquid chromatography-tandem mass spectrometry (LC–MS/MS). The CYP2D6*10/*10 and CYP2D6*wt/*10 groups had significantly higher Cmax and lower CL/F values than the CYP2D6*wt/*wt group. The AUCinf of CYP2D6*10/*10 and CYP2D6*wt/*10 groups were 5.18-fold and 2.25-fold higher than the CYP2D6*wt/*wt group, respectively. There were considerable variations in the Cmax and AUC values within each genotype group, and the variations were greater as the activity of CYP2D6 decreased. These results suggest that the genetic polymorphism of CYP2D6 significantly affected tolperisone pharmacokinetics and factor(s) other than CYP2D6 may also have significant effects on the pharmacokinetics of tolperisone.
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


