MAPK-ERK is a central pathway in T-cell acute lymphoblastic leukemia that drives steroid resistance
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 24
Abstract
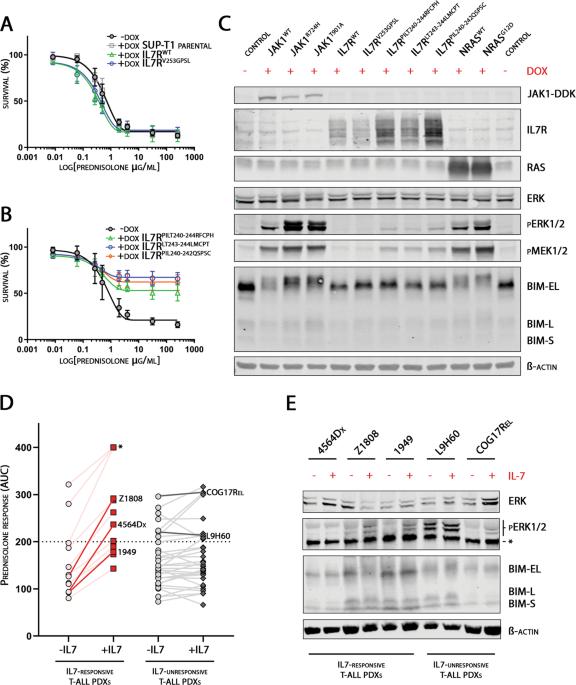
(Patho-)physiological activation of the IL7-receptor (IL7R) signaling contributes to steroid resistance in pediatric T-cell acute lymphoblastic leukemia (T-ALL). Here, we show that activating IL7R pathway mutations and physiological IL7R signaling activate MAPK-ERK signaling, which provokes steroid resistance by phosphorylation of BIM. By mass spectrometry, we demonstrate that phosphorylated BIM is impaired in binding to BCL2, BCLXL and MCL1, shifting the apoptotic balance toward survival. Treatment with MEK inhibitors abolishes this inactivating phosphorylation of BIM and restores its interaction with anti-apoptotic BCL2-protein family members. Importantly, the MEK inhibitor selumetinib synergizes with steroids in both IL7-dependent and IL7-independent steroid resistant pediatric T-ALL PDX samples. Despite the anti-MAPK-ERK activity of ruxolitinib in IL7-induced signaling and JAK1 mutant cells, ruxolitinib only synergizes with steroid treatment in IL7-dependent steroid resistant PDX samples but not in IL7-independent steroid resistant PDX samples. Our study highlights the central role for MAPK-ERK signaling in steroid resistance in T-ALL patients, and demonstrates the broader application of MEK inhibitors over ruxolitinib to resensitize steroid-resistant T-ALL cells. These findings strongly support the enrollment of T-ALL patients in the current phase I/II SeluDex trial (NCT03705507) and contributes to the optimization and stratification of newly designed T-ALL treatment regimens.
MAPK-ERK 是 T 细胞急性淋巴细胞白血病中驱动类固醇抗性的核心通路
(IL7-受体(IL7R)信号的(病理性)生理性激活导致了小儿T细胞急性淋巴细胞白血病(T-ALL)的类固醇抗性。在这里,我们发现激活的IL7R通路突变和生理性IL7R信号激活了MAPK-ERK信号,而MAPK-ERK信号通过磷酸化BIM引发类固醇抗性。通过质谱分析,我们证明了磷酸化的 BIM 与 BCL2、BCLXL 和 MCL1 的结合能力受损,从而使凋亡平衡转向存活。用MEK抑制剂处理可消除BIM的这种失活磷酸化,并恢复其与抗凋亡BCL2蛋白家族成员的相互作用。重要的是,在依赖 IL7 和不依赖 IL7 的类固醇抗性小儿 T-ALL PDX 样本中,MEK 抑制剂 selumetinib 能与类固醇产生协同作用。尽管Ruxolitinib在IL7诱导的信号传导和JAK1突变细胞中具有抗MAPK-ERK活性,但Ruxolitinib仅在依赖IL7的类固醇耐药PDX样本中与类固醇治疗产生协同作用,而在不依赖IL7的类固醇耐药PDX样本中则没有协同作用。我们的研究强调了MAPK-ERK信号在T-ALL患者类固醇耐药中的核心作用,并证明了MEK抑制剂比鲁索利替尼更广泛地应用于类固醇耐药T-ALL细胞的复敏。这些发现有力地支持了T-ALL患者加入目前的I/II期SeluDex试验(NCT03705507),并有助于优化和分层新设计的T-ALL治疗方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


