Is there a role for allogeneic hematopoietic stem cell transplantation for refractory follicular lymphoma?
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
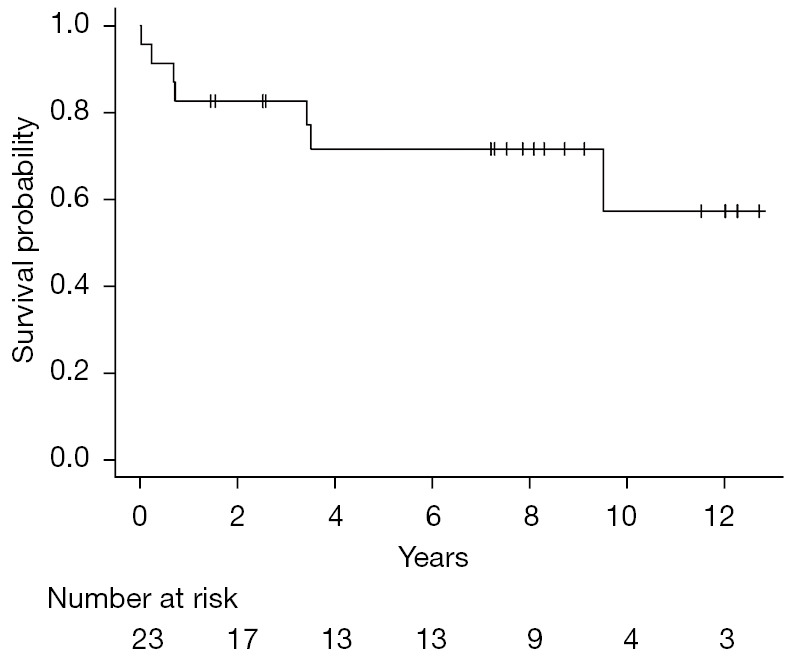
© Stem Cell Investigation. All rights reserved. Stem Cell Investig 2022;9:9 | https://dx.doi.org/10.21037/sci-2022-041 Follicular lymphoma (FL) is the most common indolent lymphoma. Although the prognosis for FL is favorable, approximately 20% of patients relapse or progress within 24 months of initial chemoimmunotherapy. Early relapse is a poor prognostic indicator with a 5-year overall survival (OS) of 50%. First relapse is usually treated with salvage chemoimmunotherapy followed by autologous stem cell transplantation (autoSCT) for chemo-responsive disease. Patients who have chemo-resistant disease or are unable to tolerate autoSCT, have a dismal prognosis. Allogeneic SCT (alloSCT) is not routinely recommended for FL patients due to the high treatment related mortality (TRM) with myeloablative conditioning regimen (MAC). The development of reduced intensity regimen (RIC) and non-myeloablative regimen (NMA) has markedly reduced TRM in alloSCT. However, the role of alloSCT vs. autoSCT in the treatment of relapsed/refractory FL (R/R FL) remains unclear. Published in Clinical Cancer Research in August 2021, the MD Anderson group retrospectively analyzed the outcomes of R/R FL patients who underwent either alloSCT or autoSCT and concluded that patients who received alloSCT had superior outcomes to those who received autoSCT (1). Khouri et al. group evaluated 194 patients with R/R FL who received NMA alloSCT (n=98) vs. autoSCT (n=96) at the MD Anderson Cancer Center between January, 2000 and December, 2017. The median follow-up was 108 months for alloSCT and 102 months for autoSCT. The OS and progression-free survival (PFS) were significantly better in patients who received alloSCT compared to autoSCT. The 8-year relapse rate was significantly lower in the alloSCT group and non-relapse mortality (NRM) between the two groups was similar. The OS for the alloSCT group was 62%, which is similar to the 61% OS reported by the large CIBMTR/EBMT study examining alloSCT in 1,567 patients with R/R FL (2). However, a similar study by the CIBMTR comparing alloSCT to autoSCT in early relapse FL reported similar OS in matched related donor (MRD) alloSCT (73%) and autoSCT (70%) and inferior OS in matched unrelated donor (MUD) alloSCT (49%). It is worth mentioning that the alloSCT group included more chemo refractory patients than the autoSCT group (40% vs. 23%) (3). It is impossible to compare the results of the referenced three trials (1-3) due to the inherent heterogeneity in methodology (single center vs. registry studies), patient, disease and transplant characteristics. In Khouri et al., the alloSCT patients were rightly highly selected with a median age of 53 years and only 24% of patients aged above 60. Additionally, most of the patients were enrolled on clinical trials, only 11% had a comorbidity index ≥3 and 16% had chemo refractory disease. Older age, poor performance status and chemo refractory disease have previously been shown to be predictors of worse outcome in R/R FL (2). The Thomas Jefferson University program has pioneered a novel alloSCT two-step approach (4). We previously reported the outcomes of the two-step alloSCT in lymphoma patients. A subgroup analysis of the indolent lymphoma patients yielded a 3-year OS of 77% (Figure 1). The majority of patients received RIC/NMA haploidentical SCT (5). Editorial Commentary
异体造血干细胞移植对难治性滤泡性淋巴瘤有作用吗?
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Stem cell investigation
Biochemistry, Genetics and Molecular Biology-Developmental Biology
CiteScore
5.80
自引率
0.00%
发文量
9
期刊介绍:
The Stem Cell Investigation (SCI; Stem Cell Investig; Online ISSN: 2313-0792) is a free access, peer-reviewed online journal covering basic, translational, and clinical research on all aspects of stem cells. It publishes original research articles and reviews on embryonic stem cells, induced pluripotent stem cells, adult tissue-specific stem/progenitor cells, cancer stem like cells, stem cell niche, stem cell technology, stem cell based drug discovery, and regenerative medicine. Stem Cell Investigation is indexed in PubMed/PMC since April, 2016.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


