Exploring the inhibitory mechanisms of indazole compounds against SAH/MTAN-mediated quorum sensing utilizing QSAR and docking.
IF 2
Q3 PHARMACOLOGY & PHARMACY
引用次数: 1
Abstract
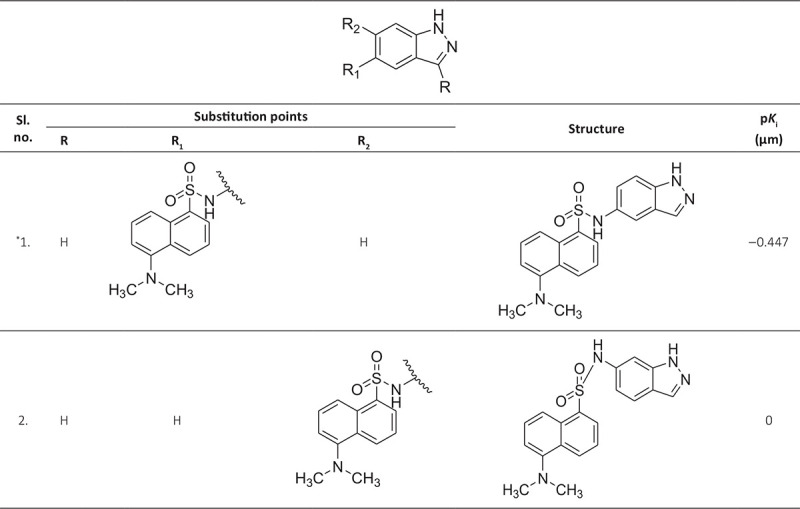
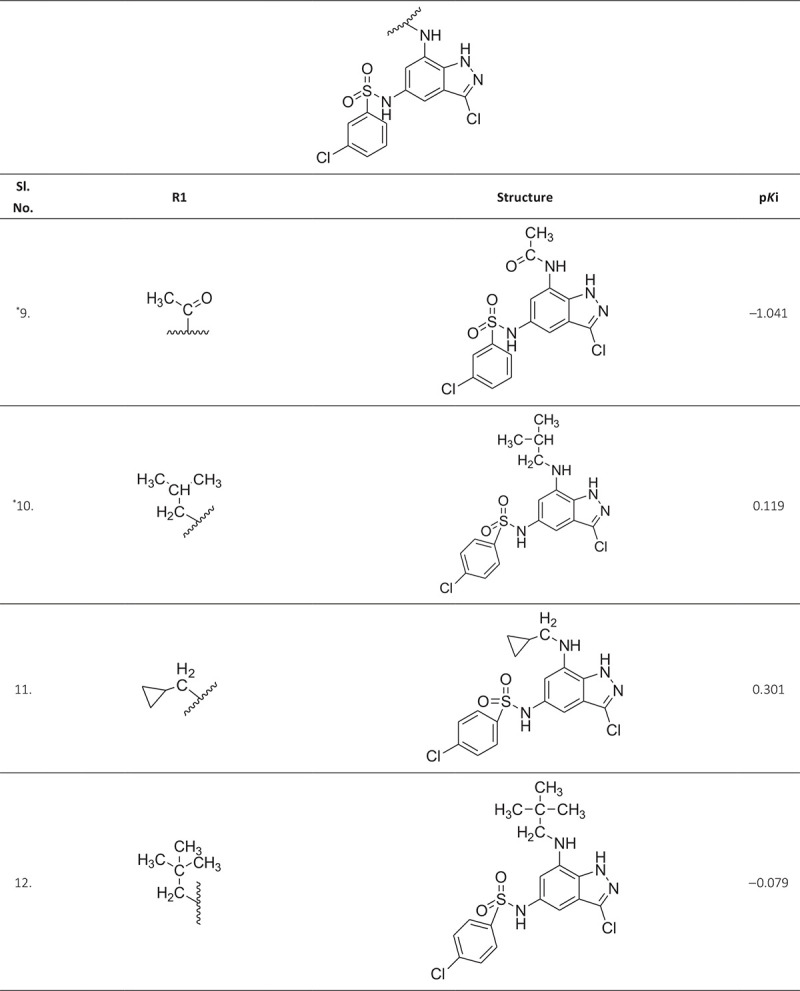
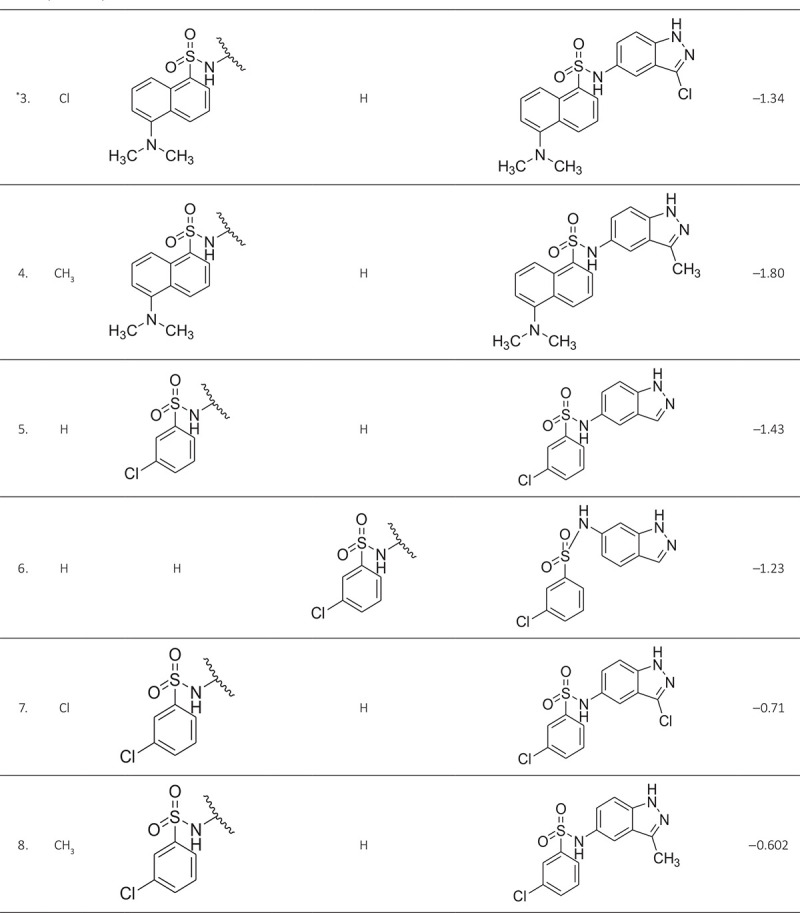
ABSTRACT The world is under the great threat of antimicrobial resistance (AMR) leading to premature deaths. Microorganisms can produce AMR via quorum sensing mechanisms utilizing S-adenosyl homocysteine/methylthioadenosine nucleosidase (SAH/MTAN) biosynthesis. But there is no specific drug developed to date to stop SAH/MTAN, which is a crucial target for the discovery of anti-quorum sensing compound. It has been shown that indazole compounds cause inhibition of SAH/MTAN-mediated quorum sensing, but the biochemical mechanisms have not yet been explored. Therefore, in this original research, an attempt has been made to explore essential structural features of these compounds by quantitative structure-activity relationship (QSAR) and molecular docking of indazole compounds having inhibition of SAH/MTAN-mediated quorum sensing. The validated QSAR predicted five essential descriptors and molecular docking helps to identify the active binding amino acid residues involved in ligand-receptor interactions that are responsible for producing the quorum sensing inhibitory mechanisms of indazole compounds against SAH/MTAN-mediated AMR.
利用QSAR和对接技术探索吲哚唑类化合物对SAH/ mtan介导的群体感应的抑制机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


