AHNAK2 Urinary Protein Expression as Potential Biomarker for Bladder Cancer Detection: A Pilot Study.
IF 1.1
Q4 UROLOGY & NEPHROLOGY
引用次数: 1
Abstract
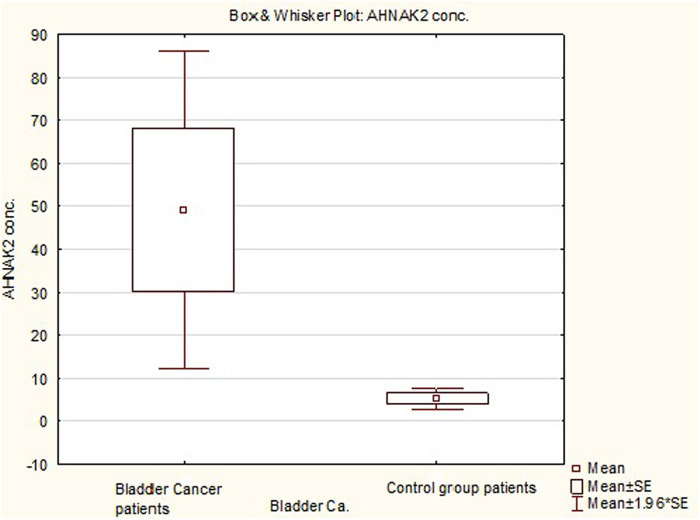
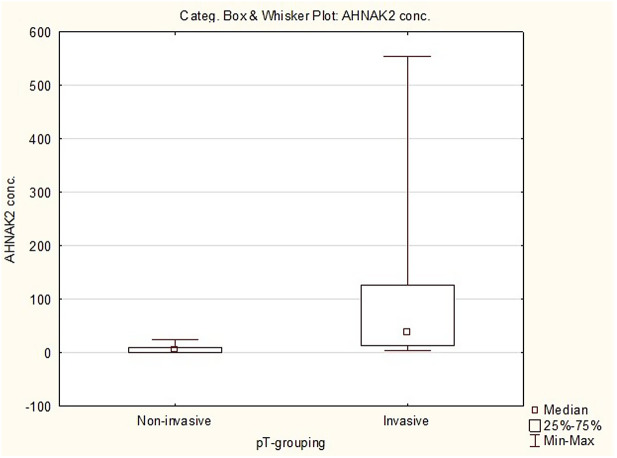
Objective: This study aimed to measure the AHNAK2 urinary levels in bladder cancer patients. Material and methods: This prospective case–control study enrolled 67 participants between January and March 2019 and were categorized into bladder cancer group (n = 37), with histologically proven bladder cancer, and control group (n = 30), with histologically verified benign lesions or with no bladder cancer indication during follow-up. Urine samples of 15 mL were collected in the mid-morning before cystoscopy/surgery and an enzyme-linked immunosorbent assay was performed as per the manufacturer’s protocol. Bladder malignancies were classified according to the World Health Organization Tumor Classification. Group’s associations were evaluated with the Student t-test, Spearman’s rank correlation, and Mann–Whitney U test, while receiver operating curve was plotted for assessing the test’s performance. Results: Mean age of the bladder cancer group was 66.41 years (standard deviation = 10.04, range = 43-82 years) and the control group was 59.67 years (standard deviation = 10.44, range = 38-77 years). All bladder cancers were of the urothelial histotype, with the following pT distribution: pTa/papillary urothelial neoplasm of low malignant potential (n = 19; 28.4%), Primary tumor (pT) in situ (n = 4; 6%), pT1 (n = 7; 10.4%), and pT≥2 (n = 7; 10.48%). Mean AHNAK2 levels were higher in bladder cancer patients 49.08 pg/mL (standard deviation = 114.91) compared to controls 5.28 pg/mL (standard deviation = 6.65), P < .05). Significant differences were noted between non-invasive bladder cancer (n = 23; mean = 7.14 pg/mL; standard deviation = 7.26) and invasive bladder cancer (n = 14; mean = 117.99 pg/mL; standard deviation = 168.08) and between non-muscle invasive bladder cancer (mean = 23.19 pg/mL; standard deviation = 66.93) and muscle-invasive bladder cancer (mean = 160.05 pg/mL; standard deviation = 199.65) (P < .001). The result of the assays was given as follows: sensitivity: 64.19%, specificity: 66.67%, positive predictive value: 22.07%, negative predictive value: 92.37%, area under curve: 0.695, and 95% CI: 0.57-0.82. Conclusion: AHNAK2 protein could be used as bladder cancer surveillance biomarker. The inclusion of AHNAK2 levels in stratification nomograms might reduce the number of unnecessary cystoscopies.

AHNAK2尿蛋白表达作为膀胱癌检测的潜在生物标志物:一项初步研究
目的:检测膀胱癌患者尿中AHNAK2水平。材料和方法:本前瞻性病例对照研究于2019年1月至3月招募了67名参与者,分为组织学证实的膀胱癌组(n=37)和对照组(n=30),随访期间组织学证实的良性病变或无膀胱癌指征。在膀胱镜检查/手术前上午收集15 mL尿液样本,并按照制造商的方案进行酶联免疫吸附试验。膀胱恶性肿瘤按照世界卫生组织肿瘤分类进行分类。采用学生t检验、Spearman秩相关检验和Mann-Whitney U检验评价组间关联,绘制受试者工作曲线评价检验效果。结果:膀胱癌组患者平均年龄为66.41岁(标准差=10.04,范围=43 ~ 82岁),对照组患者平均年龄为59.67岁(标准差=10.44,范围=38 ~ 77岁)。所有膀胱癌均为尿路上皮组织型,pT分布如下:pTa/乳头状尿路上皮肿瘤,低恶性潜能(n=19;28.4%),原发肿瘤(pT)原位(n=4;6%), pT1 (n=7;10.4%),且pT≥2 (n=7;10.48%)。膀胱癌患者的AHNAK2平均水平为49.08 pg/mL(标准差=114.91),高于对照组的5.28 pg/mL(标准差=6.65),P < 0.05。非侵袭性膀胱癌(n=23;意味着= 7.14 pg / mL;标准偏差=7.26)和浸润性膀胱癌(n=14;意味着= 117.99 pg / mL;标准偏差=168.08)和非肌性浸润性膀胱癌(平均=23.19 pg/mL;标准偏差=66.93)和肌肉浸润性膀胱癌(平均=160.05 pg/mL;标准差=199.65)(P < 0.001)。结果:敏感性为64.19%,特异性为66.67%,阳性预测值为22.07%,阴性预测值为92.37%,曲线下面积为0.695,95% CI为0.57 ~ 0.82。结论:AHNAK2蛋白可作为膀胱癌监测标志物。在分层图中包含AHNAK2水平可能会减少不必要的膀胱镜检查次数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Turkish journal of urology
Medicine-Urology
CiteScore
2.10
自引率
0.00%
发文量
53
期刊介绍:
The aim of the Turkish Journal of Urology is to contribute to the literature by publishing scientifically high-quality research articles as well as reviews, editorials, letters to the editor and case reports. The journal’s target audience includes, urology specialists, medical specialty fellows and other specialists and practitioners who are interested in the field of urology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


