Nickel Carbene-Mediated One-Carbon Homologative γ-Butyrolactonization
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 1
Abstract
In this report, we present a highly efficient approach for the synthesis of β,γ-disubstituted γ-butyrolactone motifs. This newly developed strategy is based on the combination of a diastereoselective aldol and a nickel carbene-mediated γ-butyrolactonization and uses an effective intramolecular ring closure to rapidly access a range of functionalized chiral γ-butyrolactones. This single-step approach was applied to produce straightforward asymmetric syntheses of (−)-talaumidin methyl ether, (+)-veraguensin, and (+)-dubiusamine A and a formal synthesis of (+)-phaseolinic acid as one of the shortest syntheses disclosed to date.
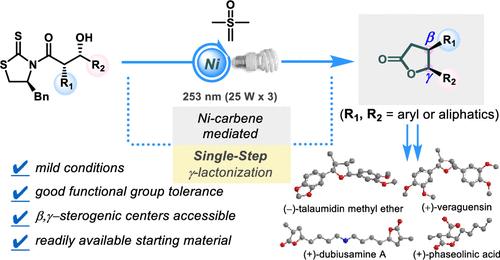
镍碳介导的一碳同源γ-丁内酯化
在这篇报道中,我们提出了一种高效合成β,γ-二取代γ-丁内酯基序的方法。这种新开发的策略是基于非对映选择性醛醇和镍碳介导的γ-丁内酯化的结合,并使用有效的分子内环关闭来快速获得一系列功能化的手性γ-丁内酯。这种单步方法被应用于直接不对称合成(−)-talaumidin甲基醚,(+)- veraguenin和(+)-dubiusamine A和(+)-phaseolinic酸的正式合成,这是迄今为止公开的最短合成之一。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


