Regulatory fine-tuning of mcr-1 increases bacterial fitness and stabilises antibiotic resistance in agricultural settings
IF 10.8
1区 环境科学与生态学
Q1 ECOLOGY
引用次数: 0
Abstract
Antibiotic resistance tends to carry fitness costs, making it difficult to understand how resistance can be maintained in the absence of continual antibiotic exposure. Here we investigate this problem in the context of mcr-1, a globally disseminated gene that confers resistance to colistin, an agricultural antibiotic that is used as a last resort for the treatment of multi-drug resistant infections. Here we show that regulatory evolution has fine-tuned the expression of mcr-1, allowing E. coli to reduce the fitness cost of mcr-1 while simultaneously increasing colistin resistance. Conjugative plasmids have transferred low-cost/high-resistance mcr-1 alleles across an incredible diversity of E. coli strains, further stabilising mcr-1 at the species level. Regulatory mutations were associated with increased mcr-1 stability in pig farms following a ban on the use of colistin as a growth promoter that decreased colistin consumption by 90%. Our study shows how regulatory evolution and plasmid transfer can combine to stabilise resistance and limit the impact of reducing antibiotic consumption.
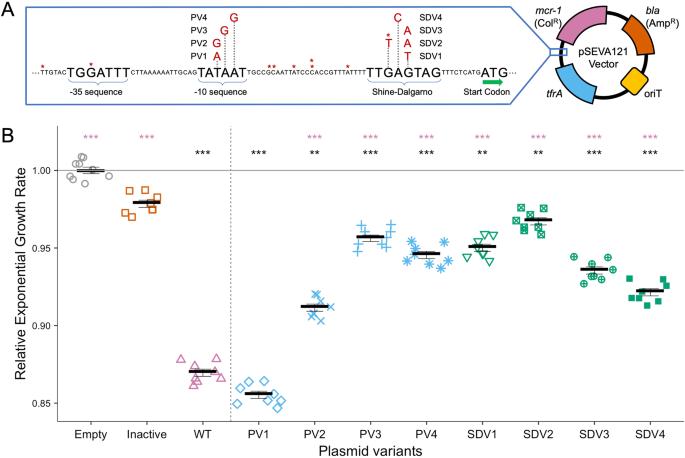
mcr-1的调节微调增加了细菌的适应度,并稳定了农业环境中的抗生素耐药性。
抗生素耐药性往往会带来健身成本,因此很难理解在没有持续接触抗生素的情况下如何保持耐药性。在这里,我们在mcr-1的背景下研究了这个问题,mcr-1是一种全球传播的基因,对粘菌素产生耐药性,粘菌素是一种农业抗生素,是治疗耐多药感染的最后手段。在这里,我们发现调节进化已经微调了mcr-1的表达,使大肠杆菌能够降低mcr-1适应度成本,同时增加粘菌素的耐药性。结合质粒已经在令人难以置信的大肠杆菌菌株多样性中转移了低成本/高抗性的mcr-1等位基因,从而在物种水平上进一步稳定了mcr-1。在禁止使用粘菌素作为生长促进剂使粘菌素消耗量减少90%后,调控突变与养猪场mcr-1稳定性增加有关。我们的研究表明,调节进化和质粒转移是如何结合起来稳定耐药性并限制减少抗生素消费的影响的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ISME Journal
环境科学-生态学
CiteScore
22.10
自引率
2.70%
发文量
171
审稿时长
2.6 months
期刊介绍:
The ISME Journal covers the diverse and integrated areas of microbial ecology. We encourage contributions that represent major advances for the study of microbial ecosystems, communities, and interactions of microorganisms in the environment. Articles in The ISME Journal describe pioneering discoveries of wide appeal that enhance our understanding of functional and mechanistic relationships among microorganisms, their communities, and their habitats.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


