Venetoclax plus hypomethylating agents in DDX41-mutated acute myeloid leukaemia and myelodysplastic syndrome: Mayo Clinic series on 12 patients
IF 5.1
2区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Venetoclax (VEN) is an FDA-approved selective inhibitor of B-cell leukaemia/lymphoma-2 (BCL-2), used for treating elderly or unfit acute myeloid leukaemia (AML) patients unable to undergo intensive chemotherapy. Combining VEN with hypomethylating agents (HMAs) has shown impressive response rates in high-risk myelodysplastic syndromes (MDS) and relapsed/refractory AML. However, the efficacy of VEN and HMAs in treating DDX41-mutated (mDDX41) MDS/AML patients remains uncertain. Despite the favourable prognostic nature of mDDX41 MDS/AML patients, there is a lack of clinical experience regarding their response to different treatment regimens, leading to an unknown optimal therapeutic approach.
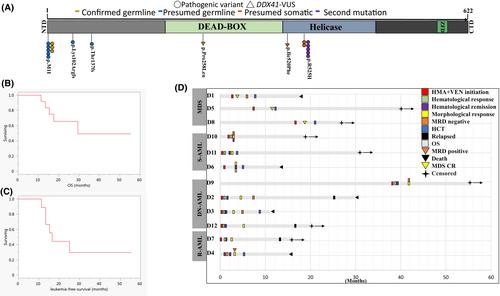
Venetoclax 加用低甲基化药物治疗 DDX41 突变的急性髓性白血病和骨髓增生异常综合征:梅奥诊所对 12 名患者的系列研究。
Venetoclax(VEN)是美国食品及药物管理局(FDA)批准的一种B细胞白血病/淋巴瘤-2(BCL-2)选择性抑制剂,用于治疗无法接受强化化疗的老年或体质不佳的急性髓性白血病(AML)患者。在高危骨髓增生异常综合征(MDS)和复发/难治性急性髓性白血病(AML)患者中,VEN与低甲基化药物(HMAs)的联合治疗显示出令人印象深刻的应答率。然而,VEN和HMAs治疗DDX41突变(mDDX41)MDS/AML患者的疗效仍不确定。尽管mDDX41 MDS/AML患者的预后较好,但缺乏有关他们对不同治疗方案反应的临床经验,导致最佳治疗方法不明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.60
自引率
4.60%
发文量
565
审稿时长
1 months
期刊介绍:
The British Journal of Haematology publishes original research papers in clinical, laboratory and experimental haematology. The Journal also features annotations, reviews, short reports, images in haematology and Letters to the Editor.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


