Integration of peripheral blood- and tissue-based biomarkers of response to immune checkpoint blockade in urothelial carcinoma
IF 5.6
2区 医学
Q1 ONCOLOGY
Rami S Vanguri, James W Smithy, Yanyun Li, Mingqiang Zhuang, Colleen A Maher, Nathaniel Aleynick, Xiyu Peng, Hikmat Al-Ahmadie, Samuel A Funt, Jonathan E Rosenberg, Gopa Iyer, Dean Bajorin, James C Mathews, Saad Nadeem, Katherine S Panageas, Ronglai Shen, Margaret K Callahan, Travis J Hollmann
下载PDF
{"title":"Integration of peripheral blood- and tissue-based biomarkers of response to immune checkpoint blockade in urothelial carcinoma","authors":"Rami S Vanguri, James W Smithy, Yanyun Li, Mingqiang Zhuang, Colleen A Maher, Nathaniel Aleynick, Xiyu Peng, Hikmat Al-Ahmadie, Samuel A Funt, Jonathan E Rosenberg, Gopa Iyer, Dean Bajorin, James C Mathews, Saad Nadeem, Katherine S Panageas, Ronglai Shen, Margaret K Callahan, Travis J Hollmann","doi":"10.1002/path.6197","DOIUrl":null,"url":null,"abstract":"<p>As predictive biomarkers of response to immune checkpoint inhibitors (ICIs) remain a major unmet clinical need in patients with urothelial carcinoma (UC), we sought to identify tissue-based immune biomarkers of clinical benefit to ICIs using multiplex immunofluorescence and to integrate these findings with previously identified peripheral blood biomarkers of response. Fifty-five pretreatment and 12 paired on-treatment UC specimens were identified from patients treated with nivolumab with or without ipilimumab. Whole tissue sections were stained with a 12-plex mIF panel, including CD8, PD-1/CD279, PD-L1/CD274, CD68, CD3, CD4, FoxP3, TCF1/7, Ki67, LAG-3, MHC-II/HLA-DR, and pancytokeratin+SOX10 to identify over three million cells. Immune tissue densities were compared to progression-free survival (PFS) and best overall response (BOR) by RECIST version 1.1. Correlation coefficients were calculated between tissue-based and circulating immune populations. The frequency of intratumoral CD3<sup>+</sup>LAG-3<sup>+</sup> cells was higher in responders compared to nonresponders (<i>p</i> = 0.0001). LAG-3<sup>+</sup> cellular aggregates were associated with response, including CD3<sup>+</sup>LAG-3<sup>+</sup> in proximity to CD3<sup>+</sup> (<i>p</i> = 0.01). Exploratory multivariate modeling showed an association between intratumoral CD3<sup>+</sup>LAG-3<sup>+</sup> cells and improved PFS independent of prognostic clinical factors (log HR −7.0; 95% confidence interval [CI] −12.7 to −1.4), as well as established biomarkers predictive of ICI response (log HR −5.0; 95% CI −9.8 to −0.2). Intratumoral LAG-3<sup>+</sup> immune cell populations warrant further study as a predictive biomarker of clinical benefit to ICIs. Differences in LAG-3<sup>+</sup> lymphocyte populations across the intratumoral and peripheral compartments may provide complementary information that could inform the future development of multimodal composite biomarkers of ICI response. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 3","pages":"349-360"},"PeriodicalIF":5.6000,"publicationDate":"2023-09-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6197","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6197","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
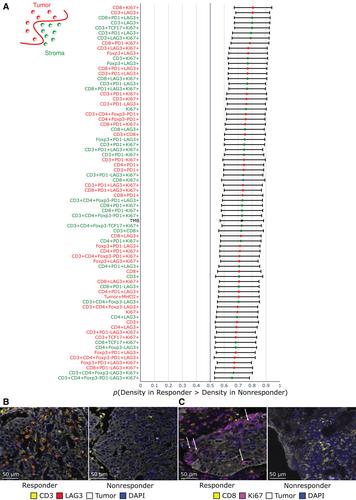
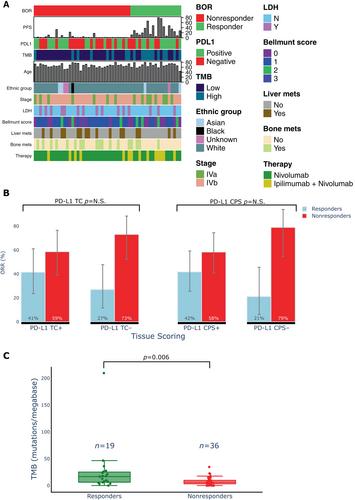
As predictive biomarkers of response to immune checkpoint inhibitors (ICIs) remain a major unmet clinical need in patients with urothelial carcinoma (UC), we sought to identify tissue-based immune biomarkers of clinical benefit to ICIs using multiplex immunofluorescence and to integrate these findings with previously identified peripheral blood biomarkers of response. Fifty-five pretreatment and 12 paired on-treatment UC specimens were identified from patients treated with nivolumab with or without ipilimumab. Whole tissue sections were stained with a 12-plex mIF panel, including CD8, PD-1/CD279, PD-L1/CD274, CD68, CD3, CD4, FoxP3, TCF1/7, Ki67, LAG-3, MHC-II/HLA-DR, and pancytokeratin+SOX10 to identify over three million cells. Immune tissue densities were compared to progression-free survival (PFS) and best overall response (BOR) by RECIST version 1.1. Correlation coefficients were calculated between tissue-based and circulating immune populations. The frequency of intratumoral CD3+ LAG-3+ cells was higher in responders compared to nonresponders (p = 0.0001). LAG-3+ cellular aggregates were associated with response, including CD3+ LAG-3+ in proximity to CD3+ (p = 0.01). Exploratory multivariate modeling showed an association between intratumoral CD3+ LAG-3+ cells and improved PFS independent of prognostic clinical factors (log HR −7.0; 95% confidence interval [CI] −12.7 to −1.4), as well as established biomarkers predictive of ICI response (log HR −5.0; 95% CI −9.8 to −0.2). Intratumoral LAG-3+ immune cell populations warrant further study as a predictive biomarker of clinical benefit to ICIs. Differences in LAG-3+ lymphocyte populations across the intratumoral and peripheral compartments may provide complementary information that could inform the future development of multimodal composite biomarkers of ICI response. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
尿路上皮癌免疫检查点阻断反应的外周血和组织生物标志物的整合
由于对免疫检查点抑制剂(ICIs)反应的预测性生物标志物仍然是尿路上皮癌(UC)患者未满足的主要临床需求,我们试图使用多重免疫荧光鉴定对ICIs具有临床益处的基于组织的免疫生物标志物,并将这些发现与先前鉴定的外周血反应生物标志物相结合。从接受nivolumab治疗或不接受ipilimumab治疗的患者中鉴定出55份预处理UC标本和12份配对治疗UC标本。全组织切片用12-复合mIF板染色,包括CD8、PD-1/CD279、PD-L1/CD274、CD68、CD3、CD4、FoxP3、TCF1/7、Ki67、LAG-3、MHC-II/HLA-DR和全细胞角蛋白+SOX10,以鉴定超过300万个细胞。通过RECIST 1.1版将免疫组织密度与无进展生存期(PFS)和最佳总反应(BOR)进行比较。计算基于组织的免疫群体和循环免疫群体之间的相关系数。肿瘤内CD3+LAG-3+细胞在应答者中的频率高于无应答者(p = 0.0001)。LAG-3+细胞聚集体与反应相关,包括CD3+LAG-3+接近CD3+(p = 0.01)。探索性多变量建模显示,肿瘤内CD3+LAG-3+细胞与PFS改善之间存在相关性,而与预后临床因素无关(log HR−7.0;95%置信区间[CI]−12.7至−1.4),以及已建立的预测ICI反应的生物标志物(log HR−5.0;95%CI−9.8至−0.2)。肿瘤内LAG-3+免疫细胞群作为ICIs临床益处的预测生物标志物值得进一步研究。肿瘤内和外周区LAG-3+淋巴细胞群的差异可能提供互补信息,为ICI反应的多模式复合生物标志物的未来发展提供信息。©2023作者。病理学杂志由John Wiley&;代表大不列颠及爱尔兰病理学会的Sons有限公司。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
来源期刊
期刊介绍:
The Journal of Pathology aims to serve as a translational bridge between basic biomedical science and clinical medicine with particular emphasis on, but not restricted to, tissue based studies. The main interests of the Journal lie in publishing studies that further our understanding the pathophysiological and pathogenetic mechanisms of human disease.
The Journal of Pathology welcomes investigative studies on human tissues, in vitro and in vivo experimental studies, and investigations based on animal models with a clear relevance to human disease, including transgenic systems.
As well as original research papers, the Journal seeks to provide rapid publication in a variety of other formats, including editorials, review articles, commentaries and perspectives and other features, both contributed and solicited.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


