Gene augmentation for autosomal dominant retinitis pigmentosa using rhodopsin genomic loci nanoparticles in the P23H+/− knock-in murine model
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
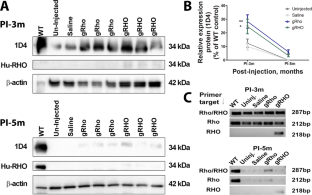
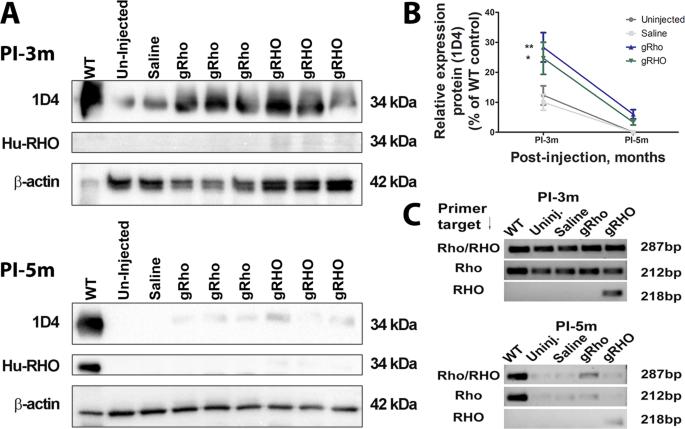
Gene therapy for autosomal dominant retinitis pigmentosa (adRP) is challenged by the dominant inheritance of the mutant genes, which would seemingly require a combination of mutant suppression and wild-type replacement of the appropriate gene. We explore the possibility that delivery of a nanoparticle (NP)-mediated full-length mouse genomic rhodopsin (gRho) or human genomic rhodopsin (gRHO) locus can overcome the dominant negative effects of the mutant rhodopsin in the clinically relevant P23H+/−-knock-in heterozygous mouse model. Our results demonstrate that mice in both gRho and gRHO NP-treated groups exhibit significant structural and functional recovery of the rod photoreceptors, which lasted for 3 months post-injection, indicating a promising reduction in photoreceptor degeneration. We performed miRNA transcriptome analysis using next generation sequencing and detected differentially expressed miRNAs as a first step towards identifying miRNAs that could potentially be used as rhodopsin gene expression enhancers or suppressors for sustained photoreceptor rescue. Our results indicate that delivering an intact genomic locus as a transgene has a greater chance of success compared to the use of the cDNA for treatment of this model of adRP, emphasizing the importance of gene augmentation using a gDNA that includes regulatory elements.
在 P23H+/- 基因敲入小鼠模型中使用视网膜基因组位点纳米颗粒进行常染色体显性色素性视网膜炎的基因扩增
常染色体显性色素性视网膜炎(adRP)的基因治疗面临突变基因显性遗传的挑战,这似乎需要将突变抑制和野生型替换适当基因相结合。我们探讨了纳米粒子(NP)介导的全长小鼠基因组视黄素(gRho)或人类基因组视黄素(gRHO)基因座的传递能否克服突变视黄素在临床相关的P23H+/--knock-in杂合小鼠模型中的显性负效应。我们的研究结果表明,gRho和gRHO NP处理组小鼠的杆状光感受器在结构和功能上都有明显的恢复,这种恢复在注射后持续了3个月,表明光感受器变性有望减少。我们利用新一代测序技术进行了 miRNA 转录组分析,并检测到了差异表达的 miRNA,这是确定 miRNA 的第一步。我们的研究结果表明,与使用 cDNA 治疗这种 adRP 模型相比,将完整的基因组位点作为转基因传递成功的几率更大,这强调了使用包含调控元件的 gDNA 进行基因扩增的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


