Long-term efficacy and safety of tonic motor activation for treatment of medication-refractory restless legs syndrome: A 24-Week Open-Label Extension Study.
IF 4.9
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 2
Abstract
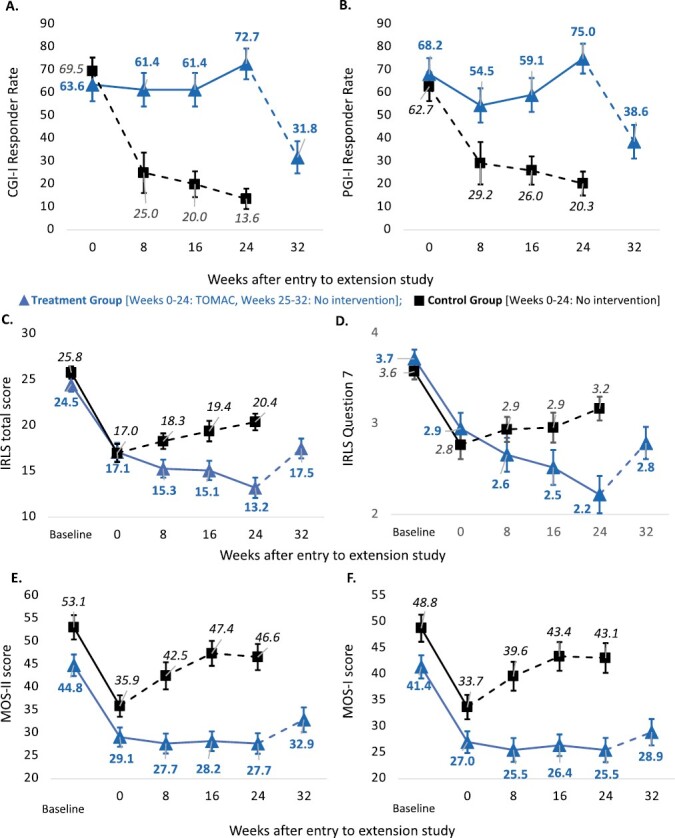
Abstract Study Objectives To evaluate long-term efficacy and safety of tonic motor activation (TOMAC) for treatment of medication-refractory moderate-to-severe primary restless legs syndrome (RLS). Methods In the parent study (RESTFUL), adults with refractory RLS were randomized to active TOMAC or sham for 4 weeks followed by 4 weeks of open-label active TOMAC. In the extension study, earlier RESTFUL completers comprised the control group (n = 59), which was followed for 24 weeks with no TOMAC intervention, and later RESTFUL completers compromised the treatment group (n = 44), which received 24 additional weeks of open-label active TOMAC followed by no intervention for 8 weeks. The primary endpoint was Clinician Global Impressions-Improvement (CGI-I) responder rate at week 24 compared to RESTFUL entry. Results CGI-I responder rate improved from 63.6% (95% CI, 49.4 to 77.9%) at RESTFUL completion to 72.7% (95% CI, 58.2 to 83.7%) at week 24 for the treatment group versus 13.6% (95% CI, 7.0 to 24.5%) at week 24 for the control group (p < 0.0001). Mean change in International RLS Rating Scale (IRLS) score improved from −7.4 (95% CI, −5.6 to −9.2) at RESTFUL completion to -11.3 points (95% CI, −8.8 to −13.9) at week 24 for the treatment group versus −5.4 (95% CI, −3.7 to −7.2) at week 24 for control group (p = 0.0001). All efficacy endpoints partially reverted during cessation of treatment. There were no grade 2 or higher device-related adverse events. Conclusions TOMAC remained safe and efficacious for >24 total weeks of treatment with partial reversion of benefits upon cessation. Clinical Trial Extension Study Evaluating NTX100 Neuromodulation System for Medication-Refractory Primary RLS; clinicaltrials.gov/ct2/show/NCT05196828; Registered at ClinicalTrials.gov with the identifier number NCT05196828.
强直性运动激活治疗药物难治性不宁腿综合征的长期疗效和安全性:一项为期24周的开放标签扩展研究。
研究目的:评价强直性运动激活(TOMAC)治疗药物难治性中重度原发性不宁腿综合征(RLS)的长期疗效和安全性。在扩展研究中,早期的RESTFUL完成者包括对照组(n=59),对照组随访24周,没有TOMAC干预,而后期的RESTFUL完成者损害了治疗组(n=44),治疗组接受了额外24周的开放标签活性TOMAC,随后8周没有干预。主要终点是与RESTFUL条目相比,临床医生在第24周的整体印象改善(CGI-I)应答率。结果:治疗组的CGI-I应答率从RESTFUL完成时的63.6%(95%CI,49.4至77.9%)提高到第24周的72.7%(95%CI,58.2至83.7%),而对照组在第24周为13.6%(95%CI)(p<0.0001)治疗组在第24周时为-8.8至-13.9,而对照组在第二十四周时为-5.4(95%CI,-3.7至-7.2)(p=0.0001)。在停止治疗期间,所有疗效终点均部分恢复。没有发生2级或更高级别的器械相关不良事件。结论:TOMAC在超过24周的治疗中仍然安全有效,停止治疗后疗效部分逆转。临床试验:评价NTX100神经调控系统治疗药物不良原发性RLS的扩展研究;clinicaltrials.gov/ct2/show/NCT051966828;在ClinicalTrials.gov注册,识别号为NCT05196828。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sleep
医学-临床神经学
CiteScore
10.10
自引率
10.70%
发文量
1134
审稿时长
3 months
期刊介绍:
SLEEP® publishes findings from studies conducted at any level of analysis, including:
Genes
Molecules
Cells
Physiology
Neural systems and circuits
Behavior and cognition
Self-report
SLEEP® publishes articles that use a wide variety of scientific approaches and address a broad range of topics. These may include, but are not limited to:
Basic and neuroscience studies of sleep and circadian mechanisms
In vitro and animal models of sleep, circadian rhythms, and human disorders
Pre-clinical human investigations, including the measurement and manipulation of sleep and circadian rhythms
Studies in clinical or population samples. These may address factors influencing sleep and circadian rhythms (e.g., development and aging, and social and environmental influences) and relationships between sleep, circadian rhythms, health, and disease
Clinical trials, epidemiology studies, implementation, and dissemination research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


