Joint use of population pharmacokinetics and machine learning for optimizing antiepileptic treatment in pediatric population.
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
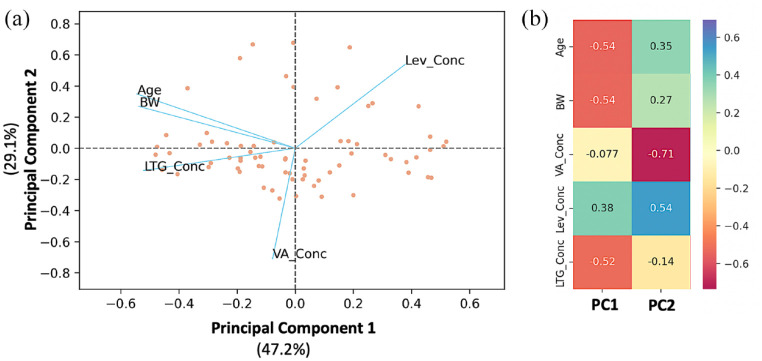
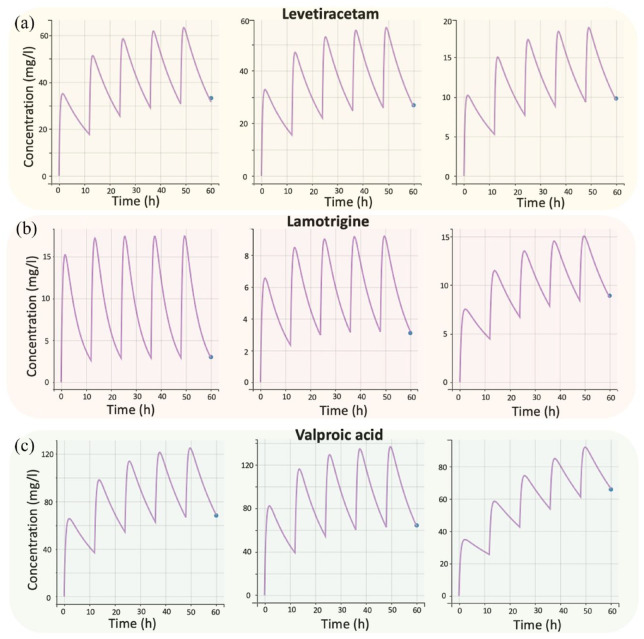
Purpose: Unpredictable drug efficacy and safety of combined antiepileptic therapy is a major challenge during pharmacotherapy decisions in everyday clinical practice. The aim of this study was to describe the pharmacokinetics of valproic acid (VA), lamotrigine (LTG), and levetiracetam (LEV) in a pediatric population using nonlinear mixed-effect modeling, while machine learning (ML) algorithms were applied to identify any relationships among the plasma levels of the three medications and patients’ characteristics, as well as to develop a predictive model for epileptic seizures. Methods: The study included 71 pediatric patients of both genders, aged 2–18 years, on combined antiepileptic therapy. Population pharmacokinetic (PopPK) models were developed separately for VA, LTG, and LEV. Based on the estimated pharmacokinetic parameters and the patients’ characteristics, three ML approaches were applied (principal component analysis, factor analysis of mixed data, and random forest). PopPK models and ML models were developed, allowing for greater insight into the treatment of children on antiepileptic treatment. Results: Results from the PopPK model showed that the kinetics of LEV, LTG, and VA were best described by a one compartment model with first-order absorption and elimination kinetics. Reliance on random forest model is a compelling vision that shows high prediction ability for all cases. The main factor that can affect antiepileptic activity is antiepileptic drug levels, followed by body weight, while gender is irrelevant. According to our study, children’s age is positively associated with LTG levels, negatively with LEV and without the influence of VA. Conclusion: The application of PopPK and ML models may be useful to improve epilepsy management in vulnerable pediatric population during the period of growth and development.

群体药代动力学和机器学习联合应用于优化儿科人群抗癫痫治疗。
目的:抗癫痫联合治疗的疗效和安全性难以预测是日常临床实践中药物治疗决策的主要挑战。本研究的目的是利用非线性混合效应模型来描述丙戊酸(VA)、拉莫三嗪(LTG)和左乙拉西坦(LEV)在儿科人群中的药代动力学,同时应用机器学习(ML)算法来确定三种药物的血浆水平与患者特征之间的关系,并建立癫痫发作的预测模型。方法:本研究纳入71例接受联合抗癫痫治疗的儿童患者,年龄2-18岁,男女均有。分别建立VA、LTG和LEV的群体药代动力学(PopPK)模型。根据估计的药代动力学参数和患者的特点,采用3种ML方法(主成分分析、混合数据因子分析和随机森林)。开发了PopPK模型和ML模型,可以更深入地了解儿童抗癫痫治疗的治疗情况。结果:PopPK模型的结果表明,LEV、LTG和VA的动力学最好地描述为具有一级吸收和消除动力学的单室模型。对随机森林模型的依赖是一个令人信服的愿景,它显示出对所有情况的高预测能力。影响抗癫痫活性的主要因素是抗癫痫药物水平,其次是体重,而性别无关。根据我们的研究,儿童年龄与LTG水平呈正相关,与LEV水平呈负相关,不受va的影响。结论:应用PopPK和ML模型可能有助于改善生长发育时期儿童易患癫痫的管理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Therapeutic Advances in Drug Safety
Medicine-Pharmacology (medical)
CiteScore
6.70
自引率
4.50%
发文量
31
审稿时长
9 weeks
期刊介绍:
Therapeutic Advances in Drug Safety delivers the highest quality peer-reviewed articles, reviews, and scholarly comment on pioneering efforts and innovative studies pertaining to the safe use of drugs in patients.
The journal has a strong clinical and pharmacological focus and is aimed at clinicians and researchers in drug safety, providing a forum in print and online for publishing the highest quality articles in this area. The editors welcome articles of current interest on research across all areas of drug safety, including therapeutic drug monitoring, pharmacoepidemiology, adverse drug reactions, drug interactions, pharmacokinetics, pharmacovigilance, medication/prescribing errors, risk management, ethics and regulation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


