Lmo4 synergizes with Fezf2 to promote direct in vivo reprogramming of upper layer cortical neurons and cortical glia towards deep-layer neuron identities.
IF 7.8
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 2
Abstract
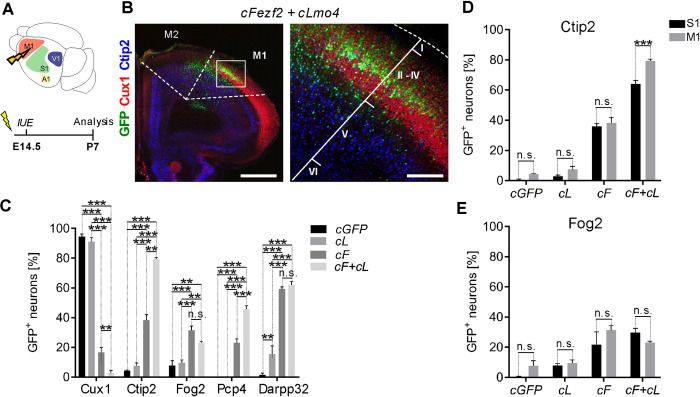
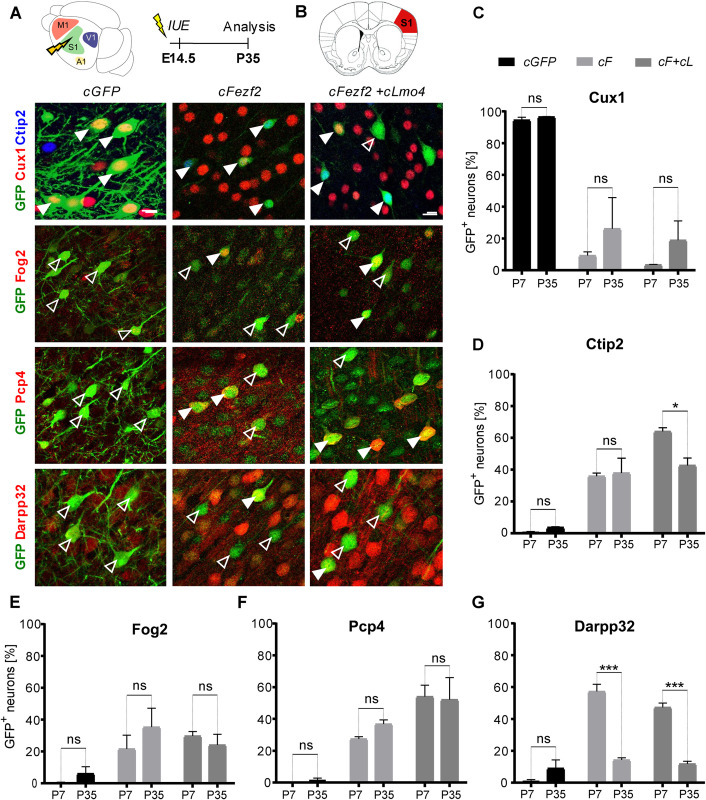
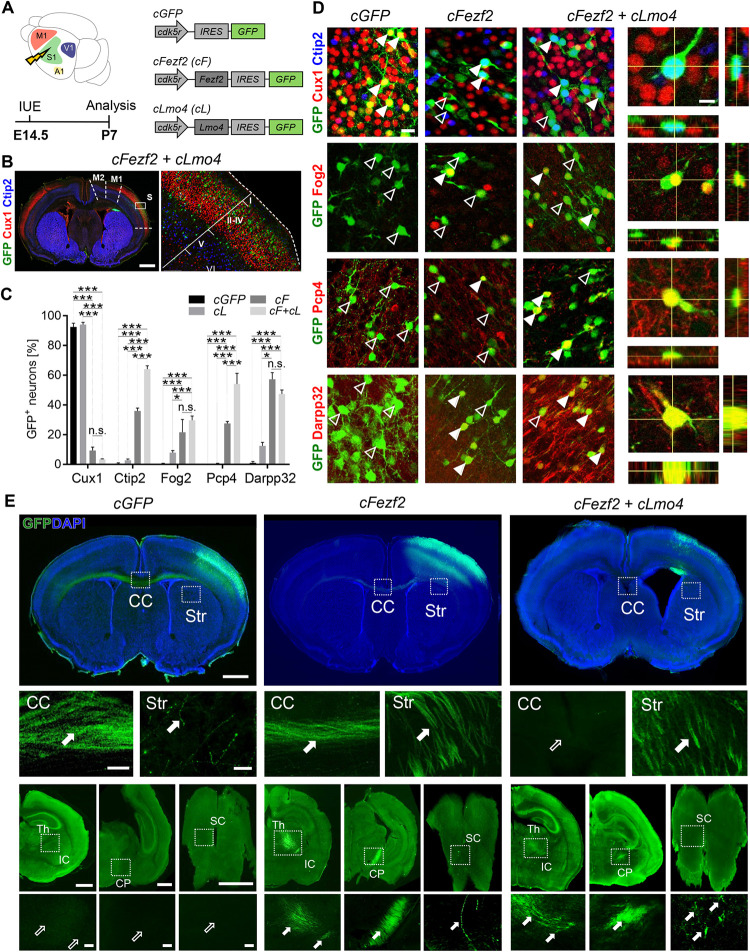
In vivo direct neuronal reprogramming relies on the implementation of an exogenous transcriptional program allowing to achieve conversion of a particular neuronal or glial cell type towards a new identity. The transcription factor (TF) Fezf2 is known for its role in neuronal subtype specification of deep-layer (DL) subcortical projection neurons. High ectopic Fezf2 expression in mice can convert both upper-layer (UL) and striatal projection neurons into a corticofugal fate, even if at low efficiency. In this study, we show that Fezf2 synergizes with the nuclear co-adaptor Lmo4 to further enhance reprogramming of UL cortical pyramidal neurons into DL corticofugal neurons, at both embryonic and early postnatal stages. Reprogrammed neurons express DL molecular markers and project toward subcerebral targets, including thalamus, cerebral peduncle (CP), and spinal cord (SC). We also show that co-expression of Fezf2 with the reprogramming factors Neurog2 and Bcl2 in early postnatal mouse glia promotes glia-to-neuron conversion with partial hallmarks of DL neurons and with Lmo4 promoting further morphological complexity. These data support a novel role for Lmo4 in synergizing with Fezf2 during direct lineage conversion in vivo.
Lmo4与Fezf2协同作用,直接在体内促进上层皮质神经元和皮层胶质向深层神经元身份的重编程。
在体内,直接神经元重编程依赖于外源性转录程序的实施,允许实现特定神经元或胶质细胞类型向新身份的转换。转录因子(TF) Fezf2因其在深层(DL)皮层下投射神经元的神经元亚型规范中的作用而闻名。Fezf2在小鼠中的高异位表达可以将上层(UL)和纹状体投射神经元转化为皮质命运,即使效率很低。在这项研究中,我们发现Fezf2与核共接头Lmo4协同作用,在胚胎和出生后早期阶段进一步增强UL皮质锥体神经元向DL皮质神经元的重编程。重编程神经元表达DL分子标记,并向丘脑、脑脚(CP)和脊髓(SC)等脑下靶点投射。我们还发现,Fezf2与重编程因子Neurog2和Bcl2在出生后早期小鼠胶质细胞中的共表达可促进胶质细胞向神经元的转化,具有DL神经元的部分特征,而与Lmo4的共表达可进一步促进形态复杂性。这些数据支持Lmo4在体内直接谱系转化过程中与Fezf2协同作用的新作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

PLoS Biology
生物-生化与分子生物学
CiteScore
14.40
自引率
2.00%
发文量
359
审稿时长
3 months
期刊介绍:
PLOS Biology is an open-access, peer-reviewed general biology journal published by PLOS, a nonprofit organization of scientists and physicians dedicated to making the world's scientific and medical literature freely accessible. The journal publishes new articles online weekly, with issues compiled and published monthly.
ISSN Numbers:
eISSN: 1545-7885
ISSN: 1544-9173
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


