EGFR激酶抑制剂的设计,合成和生物学评价,跨越正构和变构位点。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在非小细胞肺癌的治疗中,获得性耐药是一个持续的挑战,特别是在EGFR突变型中。在本研究中,我们设计、合成了同时占据EGFR正构位和变构位的新型喹唑啉和吡咯嘧啶衍生物,并对其进行了生物学评价。其中,化合物a -7被确认为潜在的EGFRL858R/T790M/C797S和EGFRDel19/T790M/C797S抑制剂。对接研究表明,化合物A-7可以同时占据EGFR的两个结合位点,并与两个区域的Met793、Lys745和Met766残基形成3个关键的h键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
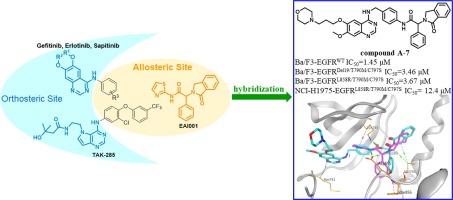
Design, synthesis and biological evaluation of EGFR kinase inhibitors that spans the orthosteric and allosteric sites
Acquired drug resistance occurred in the treatment of non-small-cell lung cancer is a persistent challenge, especially in EGFR mutant type. In this study, we present design, synthesis and biological evaluation of novel quinazoline and pyrrolopyrimidine derivatives that simultaneously occupy the orthosteric and allosteric sites of EGFR. Among them, compound A-7 was confirmed as a potential EGFRL858R/T790M/C797S and EGFRDel19/T790M/C797S inhibitor. Docking study indicated that compound A-7 could simultaneously occupy two binding sites of EGFR and form three key H-bonds with the residues Met793, Lys745 and Met766 in two regions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


