芳香残基介导的蛋白质的长程电导率。
IF 3.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 2
摘要
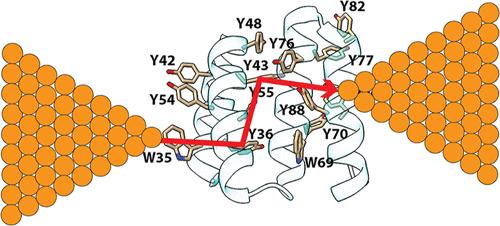
单分子测量表明,许多蛋白质在缺乏任何氧化还原辅因子的情况下,在10nm的距离上仍表现出纳米级的电导率,这意味着当电势差小于1V时,电子可以在不到一纳秒的时间内穿过整个蛋白质。这令人困惑,因为对于快速传输(即零的自由能垒),跳跃速率由大约0.8eV的重组能量决定,这将单跳的时间尺度设置为至少1μs。此外,典型金属电极的费米能量与蛋白质芳香残基的顺序氧化和还原所需的能量相去甚远,这将进一步降低跳跃电流。在这里,我们将非氧化还原活性蛋白(共有四肽重复序列)的全原子分子动力学(MD)模拟与电子转移理论相结合,以证明可以解释出乎意料的快速电子传输的分子机制。根据我们的MD模拟,充电时的能量偏移(斯托克斯偏移)产生的重组能量接近0.8 eV的传统值。然而,蛋白质对分子构型的非遍历采样导致反应重组能量,直接从静电能量波动的分布中提取,仅为~0.2 eV,其小到足以在不调用量子相干输运的情况下实现长程电导率。利用重组能的MD值,我们计算了电流随距离的衰减,这与实验一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Long-Range Conductivity in Proteins Mediated by Aromatic Residues
Single-molecule measurements show that many proteins, lacking any redox cofactors, nonetheless exhibit electrical conductance on the order of a nanosiemen over 10 nm distances, implying that electrons can transit an entire protein in less than a nanosecond when subject to a potential difference of less than 1 V. This is puzzling because, for fast transport (i.e., a free energy barrier of zero), the hopping rate is determined by the reorganization energy of approximately 0.8 eV, and this sets the time scale of a single hop to at least 1 μs. Furthermore, the Fermi energies of typical metal electrodes are far removed from the energies required for sequential oxidation and reduction of the aromatic residues of the protein, which should further reduce the hopping current. Here, we combine all-atom molecular dynamics (MD) simulations of non-redox-active proteins (consensus tetratricopeptide repeats) with an electron transfer theory to demonstrate a molecular mechanism that can account for the unexpectedly fast electron transport. According to our MD simulations, the reorganization energy produced by the energy shift on charging (the Stokes shift) is close to the conventional value of 0.8 eV. However, the non-ergodic sampling of molecular configurations by the protein results in reaction-reorganization energies, extracted directly from the distribution of the electrostatic energy fluctuations, that are only ∼0.2 eV, which is small enough to enable long-range conductivity, without invoking quantum coherent transport. Using the MD values of the reorganization energies, we calculate a current decay with distance that is in agreement with experiment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.70
自引率
0.00%
发文量
0
期刊介绍:
ACS Physical Chemistry Au is an open access journal which publishes original fundamental and applied research on all aspects of physical chemistry. The journal publishes new and original experimental computational and theoretical research of interest to physical chemists biophysical chemists chemical physicists physicists material scientists and engineers. An essential criterion for acceptance is that the manuscript provides new physical insight or develops new tools and methods of general interest. Some major topical areas include:Molecules Clusters and Aerosols; Biophysics Biomaterials Liquids and Soft Matter; Energy Materials and Catalysis

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


