一种海洋微生物组抗真菌药物靶向紧急威胁耐药真菌。
IF 44.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 84
摘要
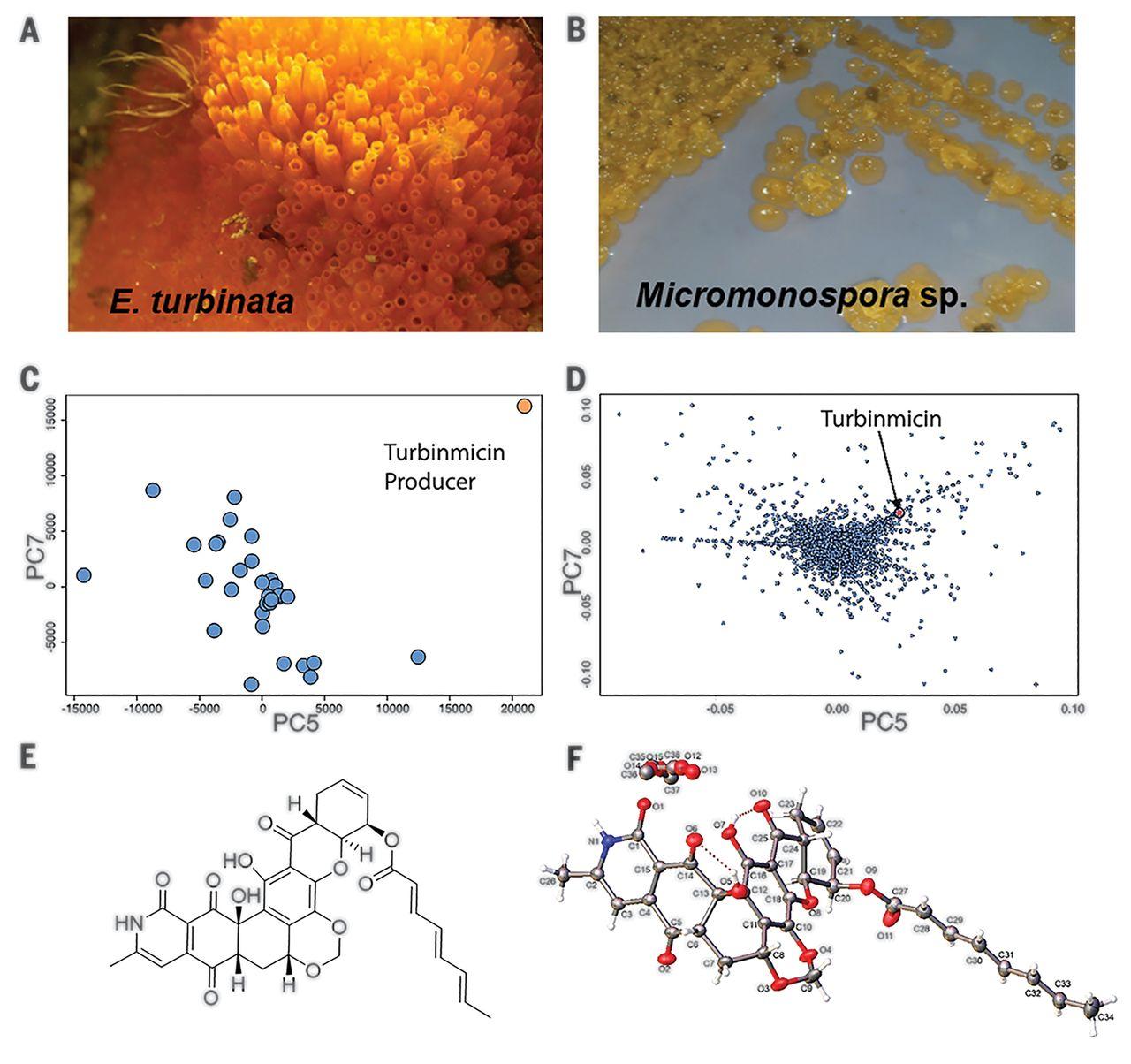
迫切需要新的抗真菌药物来解决真菌感染性疾病的出现和跨大陆传播,例如耐药念珠菌。利用海洋动物的微生物组和先进的代谢组学和基因组工具,我们发现了令人鼓舞的具有体内功效的先导抗真菌分子。最有希望的先导物turbinmicin在体外和小鼠模型中对多重耐药真菌病原体显示出强有力的疗效,具有广泛的安全指数,并通过真菌特异性的作用模式起作用,靶向水疱转运途径的Sec14。与其他抗真菌药物不同的有效性,安全性和作用方式使turbinmicin成为一种非常有前途的抗真菌药物,有助于解决破坏性的全球真菌病原体,如C. auris。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A marine microbiome antifungal targets urgent-threat drug-resistant fungi
New antifungal drugs are urgently needed to address the emergence and transcontinental spread of fungal infectious diseases, such as pandrug-resistant Candida auris. Leveraging the microbiomes of marine animals and cutting-edge metabolomics and genomic tools, we identified encouraging lead antifungal molecules with in vivo efficacy. The most promising lead, turbinmicin, displays potent in vitro and mouse-model efficacy toward multiple-drug–resistant fungal pathogens, exhibits a wide safety index, and functions through a fungal-specific mode of action, targeting Sec14 of the vesicular trafficking pathway. The efficacy, safety, and mode of action distinct from other antifungal drugs make turbinmicin a highly promising antifungal drug lead to help address devastating global fungal pathogens such as C. auris.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


