IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
通过将喹唑啉-4(3H)-酮支架、二芳基醚片段和哌嗪环结合起来的杂化策略,合成了一系列新型喹唑啉-4(3H)-酮衍生物。这些化合物对刚地弓形虫的体外活性评估表明,这一系列化合物中的大多数都表现出中等至良好的效力,IC50 值在 5.94 至 102.2 μM 之间。在合成的衍生物中,化合物 11 和 18 是最有效的抑制剂,能显著降低淋球菌的复制率,IC50 值分别为 6.33 和 5.94 μM,同时还表现出较低的细胞毒性,CC50 值分别为 285 和 59.2 μM。结构-活性关系研究表明,喹唑啉-4(3H)-酮的 N-3 位取代基对于抗淋球菌活性非常重要,而喹唑啉-4(3H)-酮的苯基和二芳基醚片段的取代基不能提高活性。侵袭和增殖试验表明,化合物 11 可以抑制寄生虫的侵袭和复制能力。对体外药效的进一步研究表明,化合物 11 对淋球菌的作用是不可逆的。在小鼠急性感染模型中进行的体内研究表明,与对照组相比,化合物 11 和 18 能显著减少寄生虫数量,同时延长受试者的存活时间。这些结果凸显了化合物 11 在开发抗弓形虫病疗法方面作为候选药物的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
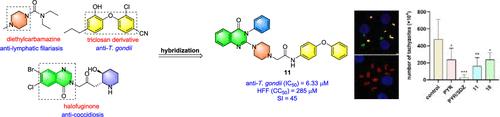
Design, Synthesis, and Structure–Activity Relationship of 2-(Piperazin-1-yl)quinazolin-4(3H)-one Derivatives as Active Agents against Toxoplasma gondii
A novel series of quinazolin-4(3H)-one derivatives were synthesized using a hybridization strategy that combined the quinazolin-4(3H)-one scaffold, the diarylether fragment, and the piperazine ring. The in vitro activity evaluation of these compounds against Toxoplasma gondii demonstrated that most of this series of compounds showed moderate to good effectiveness, with IC50 values ranging from 5.94 to 102.2 μM. Among the synthesized derivatives, compounds 11 and 18 emerged as the most potent inhibitors, significantly reducing the replication rate of T. gondii with IC50 values of 6.33 and 5.94 μM, as well as demonstrated low cytotoxicity with CC50 values of 285 and 59.2 μM, respectively. The structure–activity relationship investigation indicates that the substituent at the N-3 position of the quinazolin-4(3H)-one is important for anti-T. gondii activity while the replacements at the phenyl moiety of the quinazolin-4(3H)-one and at the diarylether fragment cannot improve activity. The invasion and proliferation assay demonstrated that compound 11 could inhibit both parasite invasion and replication ability. Further investigation of the in vitro efficacy revealed irreversible action of compound 11 against T. gondii. In vivo investigations conducted within a murine model of acute infection revealed that compounds 11 and 18 exhibited a remarkable capacity to significantly diminish the parasitic load in comparison to the control group while also extending the survival duration of the subjects. These results underscore the potential of compound 11 as a candidate for further exploration in the development of antitoxoplasmosis therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


