操纵间隙封闭纳米薄膜中亚胺连接的构型异构,实现增强型荧光传感
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
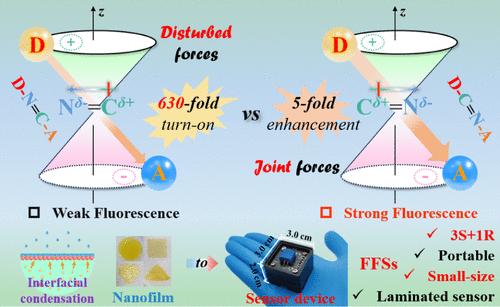
Manipulating Constitutional Isomerism of Imine Linkages in Interfacially Confined Nanofilms toward Enhanced Fluorescence Sensing
Photoluminescence efficiencies of covalent organic frameworks (COFs) are significantly restricted by electron delocalization and charge transfer among the conjugated skeletons. Two nanofilms using tetraphenylethylene and benzo[c][1,2,5]thiadiazole as the building blocks were facilely prepared via an interfacially confined condensation strategy. The distinct dipole moment orientations of imine linkages are involved in the π-delocalization of conjugated donor–acceptor systems diversely. They also played critical roles in affecting the fluorescence turn-on sensing of the obtained nanofilms for gaseous trifluoroacetic acid (TFA). The joint donor-C═N-acceptor sequence in nanofilm #2 resulted in relatively stronger fluorescence originally than that of nanofilm #1, featuring the disturbed donor-N═C-acceptor sequence. While after blowing trace TFA, the latter nanofilm #1 possessed prominent fluorescence enhancement and obvious color visualization. Comparative transient absorption observations and theoretical calculations elucidated the effective manipulation of the intramolecular charge transfer (ICT) efficiencies among the imine-linked functional skeletons. With the help of a laminated fluorescent sensor, a compact sensing platform was further integrated using optimized nanofilm #1. It exhibited good selectivity, excellent reversibility (≥50 recycles), an extraordinary detection limit (∼0.1 ppt), and a rapid recovery process to gaseous TFA. Our findings provide valuable optimizations of π-linkages in COFs and reliable fluorescent film sensors for monitoring toxic and hazardous gases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


