PSPC1通过与PU.1合作调节白血病转录程序,在AML中发挥致瘤作用
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
急性髓性白血病(AML)是一种侵袭性造血恶性肿瘤,其特点是髓细胞分化受阻和未成熟髓细胞增殖失控。在这里,我们发现副颈1(paraspeckle component 1,PSPC1)异常过表达,并与急性髓细胞白血病患者的生存率低下有关。我们利用人类急性髓细胞性白血病细胞和小鼠模型证明,PSPC1 并非正常造血所必需,但它对急性髓细胞性白血病细胞保持其白血病特征至关重要。PSPC1 的缺失会诱导多种急性髓性白血病细胞发生强分化、抑制增殖并抑制白血病的发生。从机理上讲,PSPC1通过与PU.1的染色质结合以及激活包括NDC1在内的肿瘤促进基因,调节独特的白血病转录程序,从而发挥促白血病作用。我们的研究结果揭示了 PSPC1 依赖性在急性髓细胞性白血病中独特而关键的作用,并强调了它作为急性髓细胞性白血病治疗靶点的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
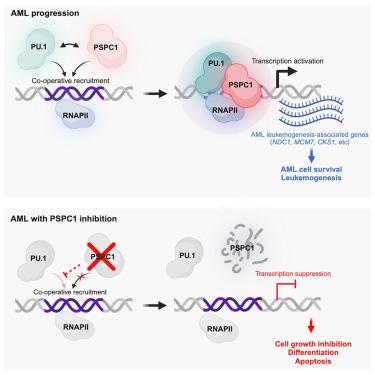
PSPC1 exerts an oncogenic role in AML by regulating a leukemic transcription program in cooperation with PU.1
Acute myeloid leukemia (AML) is an aggressive hematopoietic malignancy characterized by the blockage of myeloid cell differentiation and uncontrolled proliferation of immature myeloid cells. Here, we show that paraspeckle component 1 (PSPC1) is aberrantly overexpressed and associated with poor survival in AML patients. Using human AML cells and mouse models, we demonstrate that PSPC1 is not required for normal hematopoiesis, but it is critical and essential for AML cells to maintain their leukemic characteristics. PSPC1 loss induces robust differentiation, suppresses proliferation, and abolishes leukemogenesis in diverse AML cells. Mechanistically, PSPC1 exerts a pro-leukemia effect by regulating a unique leukemic transcription program via cooperative chromatin binding with PU.1 and activation of tumor-promoting genes, including NDC1, which is not previously implicated in AML. Our findings uncover a unique and crucial role of PSPC1 dependency in AML and highlight its potential as a promising therapeutic target for AML.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


