IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过利用核钯化引导的区域选择性氢化策略,开发出了一种硫代亚胺与烯丙基胺之间的分子间偶联,并与吡啶酰胺引导基相连,从而能够高产率地生产出一系列有价值的 N-烷基硫代亚胺。该方案具有操作简单、底物范围广的特点,也适用于苯胺类亲核物。通过放大反应和产品多样化展示了合成的实用性,从而获得了生物相关分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
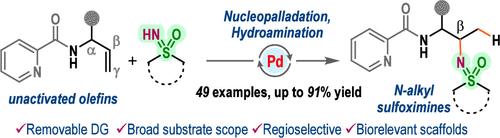
Palladium(II)-Catalyzed Regioselective Hydroamination of Allylamines to N-Alkyl Sulfoximines.
An intermolecular coupling between sulfoximines and allyl amines, linked to the picolinamide directing group, is developed by leveraging a nucleopalladation-guided regioselective hydroamination strategy, enabling the production of a range of valuable N-alkyl sulfoximines in high yields. The protocol features operational simplicity and a broad substrate scope and was also amenable to aniline nucleophiles. Synthetic utilities were showcased through scale-up reactions and product diversifications, leading to biorelevant molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


