从鱼鳞中提取的氧化石墨烯和羟基磷灰石复合材料:合成、表征和水处理应用
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在这项研究中,通过使用一种新型氧化石墨烯(GO)和正羟基磷灰石(n-HAp)复合材料的便捷吸附技术,描述了如何去除炼油废水中的碳氢化合物污染物。主要目的是合成不仅具有可持续性和生物可降解性,而且对碳氢化合物污染物具有高吸附能力和选择性的复合材料。HAp 和 GO 都是在实验室中从鱼鳞中合成的。然后通过 XRD、FTIR、SEM 和 EDX 对合成材料进行了表征。在 40 °C 和 80 分钟的反应时间内,批处理模式研究中使用的吸附剂达到了最高 97% 的有机污染物吸附率。该吸附剂的吸附活性是自发的、放热的、有用的,并且符合伪 2 阶动力学模型。使用复合吸附剂对碳氢化合物废物的吸附是在批量吸附实验模式下进行的。我们还研究了剂量、温度、时间和溶液 pH 值对吸附的影响。使用乙醇对吸附剂进行再生,然后在 70 °C 下进行干燥,以便再次使用。扫描电镜研究表明,巨大的洞穴形态粗糙、分层、起皱。通过傅立叶变换红外光谱分析发现了含氧基团和磷酸基团。将 Freundlich 和 Langmuir 动力学模型应用于吸附数据,以测量吸附行为。结果表明,Freundlich 等温线模型与实际数据之间具有很强的相关性。这项研究表明,在去除炼油废水中的有毒碳氢化合物污染物方面,这是一种高效且经济的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
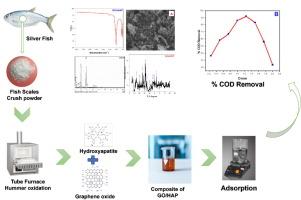
Graphene oxide and hydroxyapatite-based composite extracted from fish scales: Synthesis, characterization and water treatment application
In this study the removal of hydrocarbon pollutants from refinery waste water has been described through a convenient adsorption technique using a novel composite of graphene oxide (GO) and n-hydroxyapatite (n-HAp). The main aim was to synthesized composite which are not only sustainable and biodegradable but also exhibit high adsorption capacity and selectivity for hydrocarbon pollutants. Both the HAp and GO were synthesized from fish scales in the laboratory. The synthesized material was then characterized by XRD, FTIR, SEM, and EDX. At 40 °C and 80 min of reaction time, the adsorbent utilized in a batch mode study attained a maximum 97 % organic pollutant adsorption rate. The adsorption activity was spontaneous, exothermic, useful, and it adhered to the pseudo-2nd order kinetic model. without noticeably losing its activity, the adsorbent retained a high level of efficiency over four consecutive cycles. Adsorption of hydrocarbon wastes using composite adsorbent was carried out under batch mode of adsorption experiments. We also studied the effect of dose, temperature, time and pH of the solution on adsorption. Ethanol was used to regenerate the adsorbent, and it was then dried at 70 °C for re-use. The SEM investigation showed that the morphology of huge caves is rough, layered, and wrinkled. The oxygen and phosphate containing groups were found via FT-IR analysis. Freundlich and Langmuir kinetic models were used to measure the adsorption behavior by applying them to the adsorption data. The results demonstrated a strong correlation between the Freundlich isotherm model and the real data. This study showed a highly effective and cost-efficient methodology for the removal of toxic hydrocarbon contaminants in refinery wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


