灵活、可扩展的 4′-硫代核苷合成方法
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
4′-硫代核苷(thNAs)是一种合成核苷类似物,作为肿瘤学和病毒学领域的药物发现线索备受关注。我们在此报告了一种从头开始的 thNA 合成方法,它依赖于一种可扩展的 α-己醛乙醛的 α-氟化和醛醇反应,然后是一个简化的过程,包括羰基还原、甲磺酸酯形成和使用 NaSH 的双置换反应。我们展示了 4′-硫代-5-甲基尿苷的多克制备过程,并重点介绍了嘌呤和嘧啶噻吩核苷以及 C2′修饰噻吩核苷的生产过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
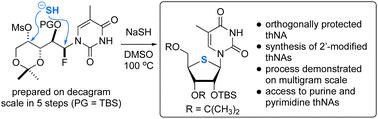
A flexible and scalable synthesis of 4′-thionucleosides
4′-Thionucleosides (thNAs) are synthetic nucleoside analogues that have attracted attention as leads for drug discovery in oncology and virology. Here we report a de novo thNA synthesis that relies on a scalable α-fluorination and aldol reaction of α-heteroaryl acetaldehydes followed by a streamlined process involving carbonyl reduction, mesylate formation and a double displacement reaction using NaSH. We demonstrate the multigram preparation of 4′-thio-5-methyluridine and highlight the production of purine and pyrimidine thNAs as well as C2′-modified thNAs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


