利用锌替代磁铁矿高效、可持续地去除和回收废水中的磷酸盐
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
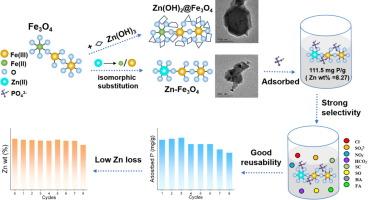
水中过量的磷会造成严重的环境问题,如藻类大量繁殖,而磷矿的过度开采则引起了人们对磷枯竭的担忧。为了高效、低成本地解决磷酸盐的去除和回收问题,我们通过在 Fe3O4 的 Fe(II)/Fe(III) 晶格中加入 Zn(II) 合成了一种 Zn 同构取代磁铁矿(Zn-Fe3O4),用于吸附废水中的磷酸盐。对初始磷酸盐浓度、吸附剂用量、吸附时间和 pH 值等关键参数进行了优化,以最大限度地吸附磷酸盐。根据 Langmuir 等温线模型,Zn-Fe3O4-8.27 % 的最大磷酸盐吸附容量为 111.5 mg P/g。选择性吸附试验表明,Zn-Fe3O4-8.27 % 对磷酸盐的亲和力高于其他干扰阴离子和溶解有机物。经过八次吸附-解吸循环后,Zn-Fe3O4-8.27 % 保持了 80 % 的初始吸附能力,成功回收了 70 % 以上的磷酸盐,仅损失了 11 % 的锌(II)。与其他锌基吸附剂相比,锌(II)的损失率大大降低,从而提高了其可再利用性。Zn(II) 和磷酸盐之间强大的静电吸引和内球络合作用是这种高效吸附和循环利用的原因。因此,Zn-Fe3O4-8.27 % 是一种用于去除废水中磷酸盐的前景看好的吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficient and sustainable phosphate removal and recovery from wastewater with Zinc-Substituted magnetite
Excess phosphorus in water causes significant environmental issues, such as algal blooms, while the over-exploitation of phosphorus ores has raised concerns about phosphorus depletion. To address both the removal and recycling of phosphate efficiently and cost-effectively, a Zn isomorphically substituted magnetite (Zn-Fe3O4) was synthesized by incorporating Zn(II) into the Fe(II)/Fe(III) lattice of Fe3O4, targeting phosphate adsorption from wastewater. Key parameters, such as initial phosphate concentration, adsorbent dosage, adsorption time, and pH were optimized to maximize phosphate adsorption. Based on the Langmuir isotherm model, Zn-Fe3O4-8.27 % demonstrated a maximum phosphate adsorption capacity of 111.5 mg P/g. Selective adsorption tests showed that Zn-Fe3O4-8.27 % had a higher affinity for phosphate than other interfering anions and dissolved organic matter. After eight adsorption–desorption cycles, Zn-Fe3O4-8.27 % retained 80 % of its initial adsorption capacity, successfully recovering over 70 % of the phosphate, with only an 11 % loss of of Zn(II). This significantly lower Zn(II) loss compared to other zinc-based adsorbents, enhancing its reusability. This high efficiency in adsorption and recycling is attributed to strong electrostatic attraction and inner-sphere complexation between Zn(II) and phosphate. Consequently, Zn-Fe3O4-8.27 % emerges as a promising adsorbent for phosphate removal from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


