YOXINTINE治疗抑郁症的疗效和安全性:双盲、随机、安慰剂对照的 2 期临床试验
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
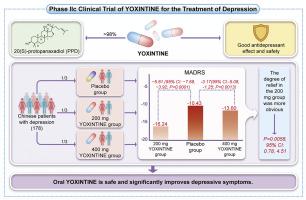
研究背景养心亭含有98%的20(S)-原人参皂苷(PPD),PPD是人参皂苷的代谢产物,具有临床前神经保护活性。研究设计这是一项多中心、双盲、随机、安慰剂对照的2期临床试验。方法该研究纳入了178名随机(1:1:1)的中国抑郁症患者。患者被随机分配接受200或400毫克剂量的YOXINTINE口服液或安慰剂,每天服用两次,连续服用8周。主要结果通过测量蒙哥马利-阿斯伯格抑郁量表(MADRS)总分的变化进行评估。所有不良反应均有记录。结果安慰剂组的MADRS总分与基线相比变化为-10.43,200毫克YOXINTINE组为-16.24,400毫克YOXINTINE组为-13.60。与安慰剂相比,200 毫克组和 400 毫克组的 MADRS 总分变化分别为-5.81(95 % CI:-7.69,-3.92;P < 0.0001)和-3.17(95 % CI:-5.08,-1.25;P = 0.0013)。结果表明,200 毫克组的 MADRS 评分降低幅度明显更大(P = 0.0058,95 % CI:0.78, 4.51)。结论口服 YOXINTINE 安全且能显著改善抑郁症状。PPD可能通过不同于单胺再摄取抑制的机制表现出抗抑郁特性。注册号ChiCTR2300070568本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficacy and safety of YOXINTINE for depression: A double-blinded, randomized, placebo-controlled, phase 2 clinical trial
Background
YOXINTINE contains >98 % of 20(S)-protopanaxadial (PPD), a metabolic product of ginsenosides with pre-clinical neuroprotective activity. Animal experiments and previous studies have shown that PPD has good antidepressant effect and safety.
Purpose
To evaluate YOXINTINE in treating depression compared with a placebo in Chinese patients.
Study Design
This was a multicenter, double-blinded, randomized, placebo-controlled, phase 2 clinical trial.
Methods
The study included 178 randomized (1:1:1) Chinese patients with depression. Patients were randomly assigned to receive oral YOXINTINE at doses of 200 or 400 mg or a placebo administered twice daily for 8 weeks. The primary outcome was assessed by measuring changes in the Montgomery–Asberg Depression Rating Scale (MADRS) total score. All adverse reactions were recorded. All demographic and baseline characteristics were comparable.
Results
The changes in MADRS total scores from baseline were −10.43 for the placebo group, −16.24 for the 200 mg YOXINTINE group, and −13.60 for the 400 mg YOXINTINE group. The differences in MADRS total score changes compared with the placebo were −5.81 (95 % CI: −7.69, −3.92; P < 0.0001) and −3.17 (95 % CI: −5.08, −1.25; P = 0.0013) for the 200 mg and 400 mg groups, respectively. The results indicated a significantly greater MADRS score reduction in the 200 mg group (P = 0.0058, 95 % CI: 0.78, 4.51). Adverse event incidence was comparable among all groups.
Conclusion
Oral YOXINTINE is safe and significantly improves depressive symptoms. PPD may exhibit antidepressant properties through mechanisms distinct from monoamine reuptake inhibition.
Registration number
ChiCTR2300070568
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


