一种细胞内细菌病原体引发了依赖 RIG-I/MDA5 的坏死细胞增多症
IF 4.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
RIG-I 和 MDA5 是类 RIG-I 受体(RLR)的成员,它们能检测到细胞质中的病毒 RNA,随后启动抗病毒免疫反应。坏死是由混合系激酶结构域样(MLKL)执行的一种程序性细胞死亡(PCD)形式,MLKL被受体相互作用蛋白激酶3(RIPK3)磷酸化后会导致坏死细胞死亡。迄今为止,在细菌感染过程中尚未观察到 RLR 与坏死之间的联系。Edwardsiella tarda 是一种可在宿主巨噬细胞中繁殖的人畜共患细菌病原体。在之前的研究中,我们发现 RIG-I 和 MDA5 是 RAW264.7 细胞对 E. tarda 感染做出反应的两个枢纽因子。本研究旨在确定E. tarda引发细胞死亡的具体形式,并探讨细菌感染背景下RIG-I/MDA5与PCD之间的关联。我们的研究结果表明,E. tarda感染诱导RAW264.7细胞发生RIPK3-MLKL介导的坏死,而不是热凋亡或细胞凋亡。同时,E. tarda促进了RIG-I/MDA5的产生,并激活了RIG-I/MDA5通路,导致IRF3磷酸化、IFN-β分泌、干扰素刺激基因(ISG)和细胞因子的表达。RIG-I 和 MDA5 都是 E. tarda 触发坏死的必要条件,也是有效抑制细胞内细菌复制的必要条件。此外,RIG-I/MDA5 对坏死的调控作用不受 I 型 IFN 或 TNF-α 信号阻断的影响。这些结果共同揭示了细胞内细菌感染可通过 RIG-I/MDA5 通路触发坏死,细菌病原体诱导的 PCD 和 RLR 之间存在错综复杂的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
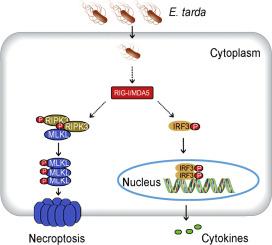
An intracellular bacterial pathogen triggers RIG-I/MDA5-dependent necroptosis
RIG-I and MDA5 are members of RIG-I-like receptors (RLRs) that detect viral RNA within the cytoplasm and subsequently initiate antiviral immune responses. Necroptosis is a form of programmed cell death (PCD) executed by mixed lineage kinase domain-like (MLKL), which, upon phosphorylation by receptor-interacting protein kinase 3 (RIPK3), causes necrotic cell death. To date, no link between RLRs and necroptosis has been observed during bacterial infection. Edwardsiella tarda is a zoonotic bacterial pathogen that can thrive in host macrophages. In a previous study, we identified RIG-I and MDA5 as two hub factors of RAW264.7 cells responsive to E. tarda infection. The present study aimed to determine the specific form of cell death triggered by E. tarda and explore the association between RIG-I/MDA5 and PCD in the context of bacterial infection. Our results showed that E. tarda infection induced RIPK3-MLKL-mediated necroptosis, rather than pyroptosis or apoptosis, in RAW264.7 cells. Meanwhile, E. tarda promoted RIG-I/MDA5 production and activated the RIG-I/MDA5 pathways that led to IRF3 phosphorylation, IFN-β secretion, and interferon-stimulated gene (ISG) and cytokine expression. Both RIG-I and MDA5 were essential for E. tarda-triggered necroptosis and required for effective inhibition of intracellular bacterial replication. Furthermore, the regulatory effect of RIG-I/MDA5 on necroptosis was not affected by type I IFN or TNF-α signaling blockage. Together these results revealed that necroptosis could be triggered by intracellular bacterial infection through the RIG-I/MDA5 pathways, and that there existed intricate interplays between PCD and RLRs induced by bacterial pathogen.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


