通过表面贴片结合诱导与明胶的络合,提高藻蓝蛋白在酸性条件下的稳定性
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
植物花青素是一种著名的蓝色天然着色剂,广泛应用于食品和饮料中。然而,植物花青素的胶体稳定性较差,在酸性条件下会从溶液中析出,导致蓝色消失。在这项研究中,明胶(0.1%-1.0%w/v)被用来保护植物花青素的胶体和颜色稳定性,同时还能增强其抗氧化活性。Zeta电位、粒度和扫描电镜结果表明,适当浓度的明胶可与植物花青素形成复合物。这是由表面贴片结合(SPB)引起的,它使植物花青素具有更强的静电斥力,从而克服了蛋白质的聚集。自由基清除实验表明,植物花青素与明胶的结合促进了植物花青素的抗氧化活性。紫外-可见吸收光谱、荧光光谱和傅立叶变换红外光谱结果表明,明胶可通过疏水作用和氢键形成稳定植物花青素和四吡咯发色团的三级结构。圆二色性进一步表明,明胶可以保留植物花青素的原生二级结构,尤其是α-螺旋结构,从而稳定发色团,保护蓝色。这项研究表明,明胶在恢复酸化的植物花青素结构和颜色方面发挥着有益的作用,有助于植物花青素作为天然色素在食品和饮料中的潜在应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
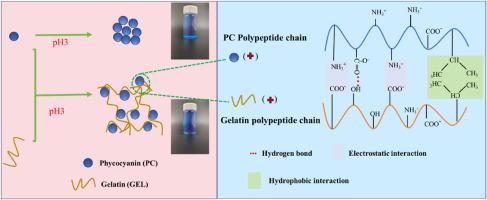
Improving stability of phycocyanin under acidic conditions by surface patch binding induced complexation with gelatin
Phycocyanin is a well-known natural colorant with a blue hue, utilized extensively in food and beverage applications. However, phycocyanin displays poor colloidal stability and precipitates from solution under acidic conditions, resulting in loss of blue color. In this study, gelatin (0.1%-1.0%w/v) was used to protect the colloidal and color stability of phycocyanin while also enhancing its antioxidant activity. Zeta-potential, particle size, and SEM results indicated that suitable concentrations of gelatin could form complexes with phycocyanin. This was induced by surface patch binding (SPB), making phycocyanin more electrostatically repulsive to overcome protein aggregation. Free radical scavenging experiments showed that phycocyanin-gelatin binding promoted the antioxidant activity of phycocyanin. UV–vis absorption, fluorescence spectra, and Fourier transform infrared spectroscopy results showed that gelatin could stabilizing the tertiary structure of phycocyanin and the tetrapyrrole chromophore through hydrophobic interactions and hydrogen bonds formation. Circular dichroism further revealed that gelatin could preserve the native secondary structure of phycocyanin, particularly the α-helix, thereby stabilizing the chromophore and protecting the blue color. This study showed that gelatin has a beneficial role in recovering acidified phycocyanin structure and color, facilitating the potential application of phycocyanin in foods and beverages as a natural pigment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


