过氧乙酸活化亚硫酸盐工艺:活性物质的生成和β-内酰胺类抗生素的降解
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
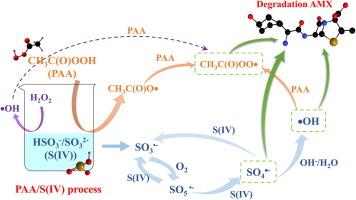
亚硫酸盐高级氧化工艺是一种新型化学氧化技术,具有很强的发展潜力,可有效产生多种活性物种,用于降解新出现的污染物(ECs)。本研究采用过乙酸活化亚硫酸盐高级氧化工艺(PAA/S(IV)工艺)降解典型的β-内酰胺类抗生素,无需额外的能量输入,也不使用重金属。在 PAA/S(IV)工艺中,79.68%的阿莫西林(AMX)在 5 分钟内被快速降解,反应 pH 值为 7.0∼9.0,降解效果最佳。在PAA/S(IV)过程中,可产生降解AMX的SO4-、-OH和CH3C(O)OO-,对AMX降解的贡献率分别为58.80%、22.24%和18.96%。HCO3-和HA清除的-OH和SO4-会影响CH3C(O)OO-的生成,进而延缓AMX的降解,而Cl-对AMX降解的影响可以忽略不计。在活性物种的作用下,β-内酰胺环断裂,并提出了五种降解途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Peroxyacetic acid activated sulfite process: Generation of reactive species and degradation of β-lactam antibiotics
Sulfite advanced oxidation process is a new chemical oxidation technology with strong development potential and can effectively produce many reactive species for the degradation of emerging contaminants (ECs). This study used the peracetic acid activated sulfite advanced oxidation process (PAA/S(IV) process) with no additional energy input and no heavy metal used to degrade typical β-lactam antibiotics. 79.68 % of amoxicillin (AMX) was rapidly degraded in the PAA/S(IV) process within 5 min, and the reaction pH of 7.0∼9.0 is the best degradation effect. In the PAA/S(IV) process, SO4•-, •OH and CH3C(O)OO• that degrade AMX can be produced, and the contribution rates to the AMX degradation were 58.80 %, 22.24 %, and 18.96 %, respectively. The presence of HCO3– and HA cleared •OH and SO4•- affected the formation of CH3C(O)OO• and then delayed the AMX degradation, while the effect of Cl- on the AMX degradation was negligible. Under the action of the reactive species, the β-lactam ring is broken, and five degradation paths are proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


