利用新型纵向辐射细胞毒性测定法,评估在辐射过程中改变分次法能否克服源自患者的胶质母细胞瘤细胞的辐射抗性。
IF 4.9
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
目的:目前对胶质母细胞瘤(GBM)的放射治疗(RT)是以恒定剂量分次(CDF)的方式进行的,这种方式没有考虑到胶质母细胞瘤的瘤内异质性和放射选择。这些因素导致了不同的治疗反应,使这一原则的疗效变得复杂。我们的研究旨在利用一种新型纵向放射细胞毒性检测方法,研究克服放射耐药性的替代剂量策略:方法:将硅内理论数学假设与体外实验策略相结合,研究替代性放射治疗方案。对具有不同辐射敏感性的患者衍生异种移植(PDX)脑肿瘤诱导细胞(BTICs)分别进行了假对照和三种方案的测试,这三种方案的名义和平均剂量相同,均为 16 Gy(分四次),但每次剂量有所改变。每 4 天进行一次常规剂量分段(CDF:4、4、4、4 Gy)或动态剂量分段(DDF)"递减"(RD:7、5、3、1 Gy)或动态剂量分段 "递增"(RU:1、3、5、7 Gy)。通过每 5 天对细胞成像收集分段间纵向数据,并在第 20 天测量终点存活率:结果:所提出的放射敏感性评估方法除了进行终点分析外,还可以进行纵向-分段间调查。事实证明,以 RD 方式提供四分剂量最能有效克服 BTIC 的获得性放射抗性(相对细胞存活率:CDF vs. RD:P):CDF与RD的对比:P 结论:通过使用室内细胞毒性预测模型和改变的放射敏感性评估,我们发现 DDF-RD 能有效地诱导具有不同放射敏感性的三种 BTIC 株系产生细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
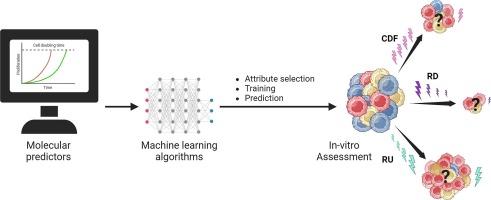
Altering fractionation during radiation overcomes radio-resistance in patient-derived glioblastoma cells assessed using a novel longitudinal radiation cytotoxicity assay
Purpose
Current radiotherapy (RT) in glioblastoma (GBM) is delivered as constant dose fractions (CDF), which do not account for intratumoral-heterogeneity and radio-selection in GBM. These factors contribute to differential treatment response complicating the therapeutic efficacy of this principle. Our study aims to investigate an alternative dosing strategy to overcome radio-resistance using a novel longitudinal radiation cytotoxicity assay.
Methods
Theoretical In-silico mathematical assumptions were combined with an in-vitro experimental strategy to investigate alternative radiation regimens. Patient-derived xenograft (PDX) brain tumor-initiating cells (BTICs) with differential radiation-sensitivities were tested individually with sham control and three regimens of the same nominal and average dose of 16 Gy (over four fractions), but with altered doses per fraction. Fractions were delivered conventionally (CDF: 4, 4, 4, 4 Gy), or as dynamic dose fractions (DDF) “ramped down” (RD: 7, 5, 3, 1 Gy), or DDF “ramped up” (RU: 1, 3, 5, 7 Gy), every 4 days. Interfraction-longitudinal data were collected by imaging cells every 5 days, and endpoint viability was taken on day 20.
Results
The proposed method of radiosensitivity assessment allows for longitudinal-interfraction investigation in addition to endpoint analysis. Delivering four-fraction doses in an RD manner proves to be most effective at overcoming acquired radiation resistance in BTICs (Relative cell viability: CDF vs. RD: P < 0.0001; Surviving fraction: CDF: vs. RD: P < 0.0001).
Conclusions
Using in-silico cytotoxicity prediction modeling and an altered radiosensitivity assessment, we show DDF-RD is effective at inducing cytotoxicity in three BTIC lines with differential radiosensitivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Radiotherapy and Oncology
医学-核医学
CiteScore
10.30
自引率
10.50%
发文量
2445
审稿时长
45 days
期刊介绍:
Radiotherapy and Oncology publishes papers describing original research as well as review articles. It covers areas of interest relating to radiation oncology. This includes: clinical radiotherapy, combined modality treatment, translational studies, epidemiological outcomes, imaging, dosimetry, and radiation therapy planning, experimental work in radiobiology, chemobiology, hyperthermia and tumour biology, as well as data science in radiation oncology and physics aspects relevant to oncology.Papers on more general aspects of interest to the radiation oncologist including chemotherapy, surgery and immunology are also published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


