大承气汤通过维持肠道血管屏障的完整性来改善肝纤维化小鼠的肝损伤。
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:严重的肝纤维化可能伴有肠道屏障损伤,如细菌性腹膜炎,这表明肠肝轴的作用不可忽视。目的:研究大承气汤(DCQD)在小鼠肝纤维化相关肠道血管屏障(GVB)损伤中的作用:研究设计:验证DCQD可降低肠道血管屏障的失衡并恢复肠道平衡,以证明DCQD通过肠肝轴发挥作用:方法:给CCL4诱导的小鼠灌胃三个剂量的DCQD,持续12周,以评估小鼠对肝脏和肠道损伤的抵抗力。免疫印迹和初级流式细胞术用于评估器官损伤;PV-1用于显示肠道血管屏障损伤;血清内毒素、粪便SCFAs和肝脏微生物群迁移用于研究肠肝轴的串联。网络药理学和RNA测序用于分析和验证DCQD的信号通路:结果:DCQD明显改善了CCL4诱导小鼠的纤维化和炎症反应,减轻了肠道渗漏,下调了PV-1,缓解了肝脏肠杆菌转位,恢复了肠道稳态,减少了固有层髓细胞的浸润。网络药理学和RNA测序结果表明,DCQD通过抑制ESR1/NF-κB/TNFα通路在肝脏中发挥抗纤维化和抗炎作用,并通过FUT2/Wnt/β-Catenin通路维持GVB稳态:结论:DCQD打破了肠肝轴的闭环损伤,改善了肝纤维化小鼠的GVB损伤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
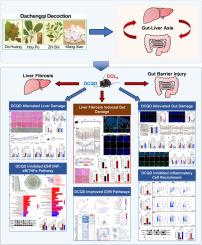
Dachengqi decoction ameliorated liver injury in liver fibrosis mice by maintaining gut vascular barrier integrity
Background
Severe liver fibrosis may be accompanied by intestinal barrier damage, such as bacterial peritonitis, suggesting that the role of the gut-liver axis is nonnegligible. Dachengqi decoction (DCQD) was reported to improve bowel movements, but whether DCQD was effective for intestinal damage caused by liver fibrosis remained unclear.
Purpose
To investigate the role of DCQD in liver fibrosis-related gut vascular barrier (GVB) damage in mice.
Study Design
DCQD was verified to reduce the imbalance of the intestinal vascular barrier and restore intestinal homeostasis to prove that DCQD acts through the gut-liver axis.
Methods
Three graded doses of DCQD were gavaged into the CCL4-induced mice for 12 weeks to evaluate the resistance to liver and intestinal damage. Immunoblotting and primary flow cytometry were used to assess organ damage; PV-1 to indicate gut vascular barrier damage; serum endotoxin, fecal SCFAs, and liver microbiota translocation to examine the gut-liver axis's crosstalk. Network pharmacology and RNA sequencing were used to analyze and verify the signaling pathway of DCQD.
Results
DCQD significantly ameliorated fibrosis and inflammatory response in the CCL4-induced mice, alleviated gut leakage, downregulated PV-1, relieved liver enterobacterial translocation, restored intestinal homeostasis, and reduced infiltration of myeloid cells in the lamina propria. Network pharmacology and RNA sequencing results indicated that DCQD exerted anti-fibrotic and anti-inflammatory effects in the liver through inhibition of the ESR1/NF-κB/TNFα pathway and maintained GVB homeostasis through the FUT2/Wnt/β-Catenin pathway.
Conclusions
DCQD broke the closed-loop damage of the gut-liver axis to improve GVB injury in mice with liver fibrosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


