双吡啶磺酰胺铑(III)复合物的生物学评价:对乳腺癌细胞线粒体动力学和细胞骨架重塑的影响
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
铑(III)配合物因其抗癌潜力而备受关注。在本研究中,我们研究了一种双吡啶磺酰胺铑(III)配合物(2)及其配体(L)对乳腺癌细胞(SKBr3)和非癌乳腺细胞(HB2)的影响。这两种化合物都能明显降低 SKBr3 细胞的氧化磷酸化(OXPHOS)和线粒体功能,而不影响 HB2 细胞。化合物 2 还增加了这两种细胞系的糖酵解,表明发生了代谢转变。线粒体的大小和形状发生了改变,尤其是在 SKBr3 细胞中。此外,这两种化合物通过破坏肌动蛋白细胞骨架组织和 Rac1/VASP 信号通路,减少了癌细胞的迁移。这些研究结果表明,联吡啶磺酰胺铑(III)复合物可选择性地损害线粒体动力学和癌细胞的迁移,而不损害健康细胞,这为了解其作用机制以及将其用作靶向抗癌疗法提供了依据。这项研究为今后的体内研究和进一步优化这些基于金属的疗法的临床应用奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
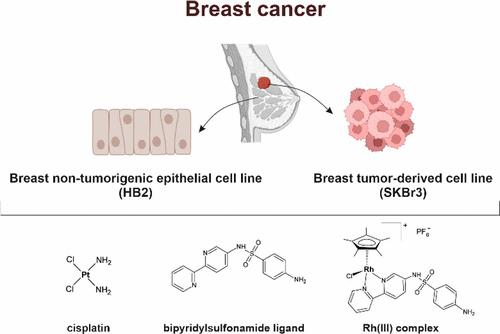
Biological Evaluation of a Rhodium(III) Bipyridylsulfonamide Complex: Effects on Mitochondrial Dynamics and Cytoskeletal Remodeling in Breast Cancer Cells
Rhodium(III) complexes have gained attention for their anticancer potential. In this study, we investigated a rhodium(III) bipyridylsulfonamide complex (2) and its ligand (L) for their effects on breast cancer (SKBr3) and noncancerous mammary cells (HB2). Both compounds significantly reduced oxidative phosphorylation (OXPHOS) and mitochondrial function in SKBr3 cells while sparing HB2 cells. Compound 2 also increased glycolysis in both lines, suggesting a metabolic shift. Mitochondrial size and shape were altered, particularly in SKBr3 cells. Additionally, both compounds reduced cancer cell migration by disrupting actin cytoskeleton organization and the Rac1/VASP signaling pathway. These findings suggest that the rhodium(III) bipyridylsulfonamide complex selectively impairs mitochondrial dynamics and cell migration in cancer cells while sparing healthy cells, providing insight into its mechanism of action and toward its use as targeted anticancer therapy. This study lays the groundwork for future in vivo studies and further optimization of these metal-based therapeutics for clinical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


