定量阐释表面防污剂与生物结合性能之间的权衡关系
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
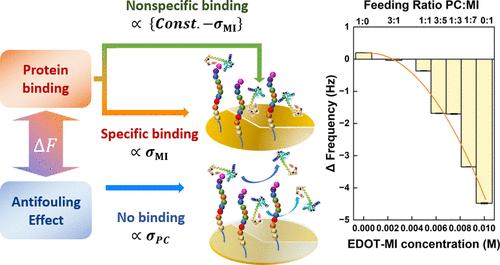
众所周知,具有高亲水性的齐聚物被广泛用于减少复杂生物溶液中生物分子的非特异性吸附。然而,这些材料也会降低目标物与肽探针之间的捕获效率。为了证明防污表面是如何影响捕获效率的,我们利用了一种基于聚(3,4-亚乙二氧基噻吩)(PEDOT)的表面,其中含有不同比例的磷酰胆碱(PEDOT-PC)和马来酰亚胺官能团,以实现防污特性和肽-蛋白质结合。作为一个模型系统,通过马来酰亚胺基团连接的肽 YWDKIKDFIGGSSSSC 被用来捕获目标蛋白质钙调蛋白(CaM)。通过使用带耗散的石英晶体微天平系统地监测蛋白质在防污和肽固定的 PEDOT 表面上的结合情况,结果表明 PEDOT-PC 既减少了肽与目标蛋白质之间的特异性结合,也降低了蛋白质在电极表面的结垢速度。根据这些发现,我们提出了一个定量分析方程。此外,我们还采用了电化学阻抗光谱法和差分脉冲伏安法来测量 CaM 溶液中阻抗的变化。数据表明,阻抗随蛋白质吸附量的增加而增加,这证实了所设计电极表面的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quantitatively Elucidating the Trade-Off between Zwitterionic Antifouling Surfaces and Bioconjugation Performance
Zwitterionic materials, known for their high hydrophilicity, are widely used to minimize the nonspecific adsorption of biomolecules in complex biological solutions. However, these materials can also reduce the capture efficiency between targets and peptide probes. To demonstrate how antifouling surfaces affect capture efficiency, we utilize a poly(3,4-ethylenedioxythiophene) (PEDOT)-based surface incorporating varying ratios of phosphorylcholine (PEDOT-PC) and maleimide functional groups to achieve both antifouling properties and peptide–protein binding. As a model system, the peptide YWDKIKDFIGGSSSSC, attached via maleimide groups, is used to capture the target protein, calmodulin (CaM). By systematically monitoring protein binding on both antifouling and peptide-immobilized PEDOT surfaces using a quartz crystal microbalance with dissipation, the results reveal that PEDOT-PC reduces both the specific binding between peptides and target proteins as well as the rate of protein fouling on the electrode surface. From these findings, we propose an equation for quantitative analysis. Furthermore, electrochemical impedance spectroscopy and differential pulse voltammetry are performed to measure the changes in the impedance in CaM solutions. The data indicate that impedance increases with protein adsorption, confirming the practical utility of the designed electrode surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


