宏观疏水性矿物滑石的亲水改性及其在浮选中的具体应用
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二乙基二硫代氨基甲酸钠(DDTC)是一种常用的捕收剂,用于提高矿物在泡沫浮选中的疏水性,但却会削弱滑石表面的疏水性。为了合理解释这种反常现象,研究人员研究了异极性表面活性剂 DDTC 的疏水烷基和亲水亲矿基团 (-NCS2-) 以及水分子与滑石 (001) 表面的相互作用。DFT 模拟发现,滑石(001)表面具有天然的疏水性,这是由粘附力(表面水)和内聚力(水-水相互作用)之间的竞争决定的。与 -NCS2- 基团和 H2O 的相互作用相比,DDTC 的疏水烷基与滑石表面的相互作用更为有利,有利于滑石表面的亲水改性。此外,吸附等温线、飞行时间二次离子质谱(ToF-SIMS)、微浮测试和接触角测量也表明,异极性表面活性剂 DDTC 在滑石表面的吸附取向差异增强了滑石表面的亲水性,导致滑石回收率下降。这项研究为杂极性表面活性剂的选择性吸附提供了重要的表面化学证据,有助于理解从滑石中高效浮选分离辉钼矿的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
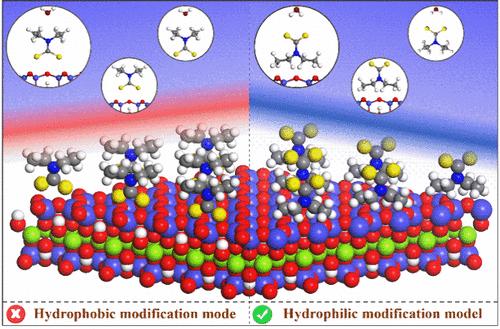
Hydrophilic Modification of Macroscopically Hydrophobic Mineral Talc and Its Specific Application in Flotation
Sodium diethyldithiocarbamate (DDTC), a common collector used to enhance the hydrophobicity of minerals in froth flotation, nevertheless weakens the hydrophobicity of the talc surface. To rationalize this anomaly, the interactions of a hydrophobic alkyl group and hydrophilic mineralophilic group (−NCS2–) of heteropolar surfactant DDTC, and a water molecule with the talc (001) surface, were investigated. Herein, DFT simulations found that the talc (001) surface features natural hydrophobicity determined by the competition between adhesion (surface water) and cohesion (water–water interactions). The interaction of the hydrophobic alkyl group of DDTC with the talc surface is more favorable compared to that of the −NCS2– group and H2O, favoring the hydrophilic modification of the talc surface. Additionally, adsorption isotherms, time-of-flight secondary ion mass spectrometry (ToF-SIMS), microflotation tests, and contact angle measurements also indicate that the differences in adsorption orientation of the heteropolar surfactant DDTC on the talc surface enhance the hydrophilicity of the talc surface, leading to a decreased recovery of the talc. This study provides crucial surface chemistry evidence for the selective adsorption of heteropolar surfactants and contributes to the understanding of the mechanism for the efficient flotation separation of molybdenite from talc.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


