用于制氢的聚羟基钾酸酯辅助光催化二氧化钛薄膜
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
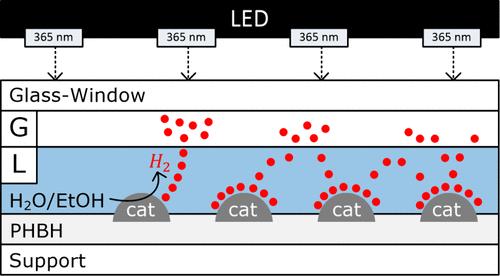
使用生物聚合物固定化二氧化钛(TiO2)进行光催化制氢是一种创新的、可持续的可再生能源发电方法。二氧化钛是一种著名的光催化剂,由于其环境友好性、丰富性和生物降解性,可固定在生物聚合物上。另一方面,为了提高二氧化钛的效率,可以通过加入铂(Pt)等助催化剂来改变其表面特性,从而改善电荷分离。在这项工作中,用铂纳米粒子(Pt1%@PC500)修饰了商用 TiO2 PC500 的表面,然后用聚羟基烷酸酯生物聚合物聚(羟基丁酸-共羟基己酸酯)(PHBH)将其固定在玻璃表面。利用各种理化技术对制备的固定化铂改性 TiO2 光催化剂进行了全面表征。研究了光催化剂薄膜的光催化活性,以乙醇为牺牲供体,通过光催化还原水制氢。详细研究了薄膜制备条件(如 PHBH 浓度、PHBH:催化剂比例和温度)对活性和稳定性的影响。应用生物聚合物 PHBH 作为粘合剂为传统的固定化方法提供了一种绿色的替代方法,并且应用 PHBH 制备出了一种稳定、活跃的光催化剂薄膜,与悬浮光催化剂相比,该薄膜的活性较低,但在六次运行中具有良好的可回收性。进行了长期光催化制氢实验。使用面积为 5.3 cm2 的 PHBH/Pt1%@PC500 薄膜,在 98 小时的连续三次运行中产生了 12 mmol 的氢气。第一次运行后,氢气生产率明显降低,这可能是由于薄膜结构发生了变化,但此后第二和第三次运行的生产率几乎保持不变。氢气是气相中的主要产物(90%),但二氧化碳(4-5%)和甲烷(4-5%)也是重要的副产品。这些副产品是使用牺牲试剂乙醇的结果。薄膜的性能结果对大规模连续制氢非常有前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Polyhydroxykanoate-Assisted Photocatalytic TiO2 Films for Hydrogen Production
The photocatalytic production of hydrogen using biopolymer-immobilized titanium dioxide (TiO2) is an innovative and sustainable approach to renewable energy generation. TiO2, a well-known photocatalyst, benefits from immobilization on biopolymers due to its environmental friendliness, abundance, and biodegradability. In another way, to boost the efficiency of TiO2, its surface properties can be modified by incorporating co-catalysts like platinum (Pt) to improve charge separation. In this work, the surface of commercial TiO2 PC500 was modified with Pt nanoparticles (Pt1%@PC500) and then immobilized on glass surfaces using polyhydroxyalkanoate biopolymer poly(hydroxybutyrate-co-hydroxyhexanoate) (PHBH). The as-prepared immobilized Pt-modified TiO2 photocatalysts were fully characterized using various physicochemical techniques. The photocatalytic activity of the photocatalyst film was investigated for photocatalytic hydrogen production through water reduction using ethanol as a sacrificial donor. The impact of the film preparation conditions, e.g., PHBH concentration, PHBH:catalyst ratio, and temperature, on activity and stability was studied in detail. The application of biopolymer PHBH as a binder provides a green alternative to conventional immobilization methods, and with the application of PHBH, a stable and active photocatalyst film that showed lower activity compared to that of the suspended photocatalyst but good recyclability in six runs was prepared. A long-term photocatalytic hydrogen production experiment was carried out. In 98 h of operation, 12 mmol of hydrogen was produced in three consecutive runs with a PHBH/Pt1%@PC500 film having an area of ∼5.3 cm2. A significantly lower hydrogen productivity was observed after the first run, possibly due to a change in film structure, but thereafter, the productivity remained almost constant for the second and third runs. Hydrogen was the main product in the gas phase (90%), but carbon dioxide (4–5%) and methane (4–5%) were obtained as important byproducts. The byproducts are a consequence of the use of the sacrificial reagent ethanol. The results of the film performance are very promising, with regard to large-scale continuous hydrogen production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


