岩石、矿物和水泥废料通过增强岩石风化去除二氧化碳的适宜性
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
有人提出,向环境中添加矿物和岩石是去除大气中二氧化碳的一种途径。当水合矿物或岩石增加碱度,促进碳酸氢盐的形成时,就会发生这一过程。在这项研究中,我们评估了常用水合岩石和矿物粉末提高碱度并与大气和浓缩二氧化碳反应的潜力。硅酸盐矿物和岩石与大气中的 CO2 反应性极小,但能适度提高碱度。玄武岩等火山岩也能释放二氧化碳。从不含二氧化碳的超基性岩中提炼出的研磨水泥和 Mg(OH)2 能显著提高碱度,并使大气中和浓缩的二氧化碳矿化。然而,水泥废料的有效性因其不同的 CaO 含量和潜在的重金属贡献而受到限制。总之,从硅酸盐中提取的 Mg(OH)2 为二氧化碳的去除和封存提供了一条很有前景的途径。通过增强岩石风化来捕获二氧化碳要求岩石和矿物土壤添加物能促进二氧化碳向碳酸氢盐的转化,通常是通过增加基质碱度来实现。在此,作者评估了水泥废料等常用水合岩石和矿物粉末提高碱度并与大气和浓缩二氧化碳反应的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
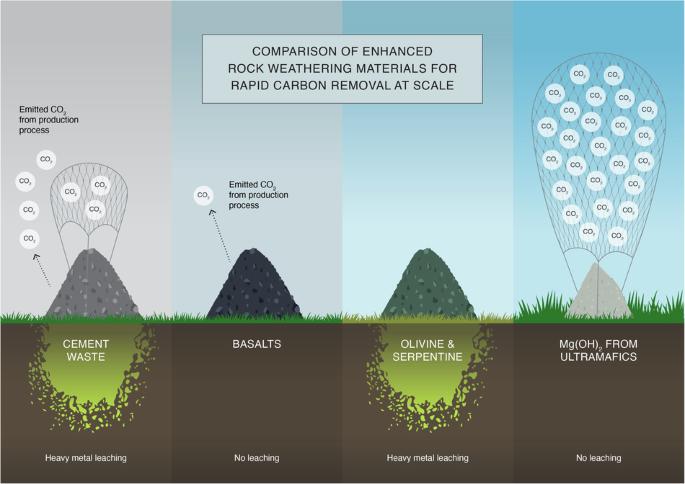
Suitability of rocks, minerals, and cement waste for CO2 removal via enhanced rock weathering
Mineral and rock additions to the environment have been proposed as a pathway to remove atmospheric CO2. This process occurs when hydrated minerals or rocks increase alkalinity, promoting the formation of bicarbonate. In this study, we evaluate the potential of commonly used hydrated rock and mineral powders to enhance alkalinity and react with both atmospheric and concentrated CO2. Silicate minerals and rocks exhibit minimal reactivity with atmospheric CO2 and provide moderate alkalinity enhancement. Volcanic rocks like basalt were shown to release CO2. Ground cement and Mg(OH)2, refined from CO2-free ultramafic rock, significantly increase alkalinity and mineralize both atmospheric and concentrated CO2. However, the effectiveness of cement waste is limit by its variable CaO content and potential heavy metal contributions. Overall, Mg(OH)2, derived from silicates, offers a promising pathway for the removal and storage of CO2. CO2 capture through enhanced rock weathering requires that rock and mineral soil additions promote CO2 to bicarbonate transformation, typically by increasing substrate alkalinity. Here, the authors evaluate the potential of commonly used hydrated rock and mineral powders such as cement waste to enhance alkalinity and react with both atmospheric and concentrated CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


