揭示离子液体混合物中 N2 溶解和扩散增强的分子机制
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
氨的合成关键取决于 N2,N2 是一种主要原料,其溶解度会影响反应动力学和产品选择性。离子液体(IL)具有结构可调的优势,可以调节 N2 的溶解度。在实际应用中,离子液体通常与其他溶剂混合,以实现反应和传输过程之间的平衡。本文通过分子动力学模拟对混合体系中 N2 的溶解和扩散行为进行了微观分析。结果表明,在四氢呋喃中引入少量三(五氟乙基)-三氟磷酸三己基(十四烷基)鏻,可显著提高 N2 的溶解度和扩散系数。过渡点受纳米空腔分数的影响,表现出明显的膨胀区、饱和区和压缩区。此外,混合体系中的 N2 溶解度对温度变化的响应趋势随摩尔浓度的变化而变化,反映了溶解焓变化的差异。这些发现为设计具有高 N2 溶解和扩散能力的混合溶剂提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
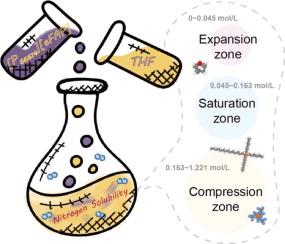
Unveiling molecular mechanisms of enhanced N2 solvation and diffusion in ionic liquid mixtures
Ammonia synthesis depends on N2 critically, a principal raw material whose solubility influences the reaction kinetics and product selectivity. Ionic liquids (ILs) possess the advantage of structure tunability to modulate N2 solubility. In practical applications, ILs are commonly mixed with other solvents to achieve a balance between reaction and transport processes. Herein, the dissolution and diffusion behaviors of N2 in mixed systems are analyzed microscopically through molecular dynamics simulations. It reveals that introducing a small quantity of trihexyl(tetradecyl)phosphonium tris(pentafluoroethyl)-trifluorophosphate into tetrahydrofuran enhances the solubility and diffusion coefficients of N2 significantly. The transition point is governed by the fraction of nanocavity, which exhibits distinct expansion, saturation, and compression zones. Furthermore, the response trend of N2 solubility in mixed systems to temperature changes varies with molar concentrations, reflecting differences in solvation enthalpy changes. These findings provide theoretical insights for the design of mixed solvents with high N2 dissolution and diffusion capabilities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


