钴催化分子内对映体选择性还原赫克反应合成手性 3-三氟甲基羰基吲哚
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文公开了一种钴催化的分子内对映选择性还原 Heck 反应。以 N-正碘芳基-2-(三氟甲基)丙烯酰胺为起点,通过使用锌/硅烷作为还原剂,获得了大量在 C3 位上带有三氟甲基四元立体中心的手性吲哚,收率中等至良好(高达 88%),对映选择性良好至极佳(高达 94%ee)。除了三氟甲基外,还获得了一些在 C3 位上带有烷基、芳基和酯基的手性羰基吲哚,尽管对映选择性相对较低(68-78% ee)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
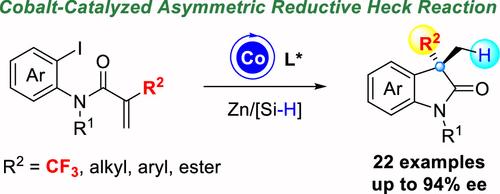
Cobalt-Catalyzed Intramolecular Enantioselective Reductive Heck Reaction toward the Synthesis of Chiral 3-Trifluoromethylated Oxindoles
Herein, a cobalt-catalyzed intramolecular enantioselective reductive Heck reaction is disclosed. Starting from N-ortho-iodoaryl-2-(trifluoromethyl)acrylamides, a plethora of chiral oxindoles bearing trifluoromethylated quaternary stereogenic centers at the C3-position are achieved in moderate to good yields (up to 88% yield) and good to excellent enantioselectivities (up to 94% ee) by employing zinc/silane as reducing agent. Other than the trifluoromethyl group, a number of chiral oxindoles bearing alkyl, aryl, and ester groups at C3-position were also obtained albeit in relatively lower enantioselectivities (68–78% ee).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


